tert-Butyl Alcohol (tBA)
CASRN 75-65-0 | DTXSID8020204
On this page:
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (7 pp, 310 K) Last Updated: 08/13/2021
| System | RfD (mg/kg-day) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Urinary | 4 x 10 -1 | Increased severity of nephropathy |
BMDL
10
(HED):
43.2
mg/kg-day |
100 | Medium |
Reference Concentration for Inhalation Exposure (RfC) (PDF) (7 pp, 310 K) Last Updated: 08/13/2021
| System | RfC (mg/m3) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Urinary | 5 | Increased severity of nephropathy |
BMCL
10
(HEC):
491
mg/m3 |
100 | Medium |
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(7 pp, 310 K)
Last Updated: 08/13/2021
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| Suggestive evidence of carcinogenic potential | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005) |
- Under EPA’s Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005), tert-butyl alcohol has “suggestive evidence of carcinogenic potential” for all routes of exposure based on some evidence in animals.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (7 pp, 310 K)
Suggestive evidence of carcinogenic potential
Oral Slope Factor:
5
x 10-4
per mg/kg-day
(OSF based on increasing thyroid tumors in mice. )
Tumor site(s): Urinary, Endocrine
Tumor type(s): Renal adenomas and carcinomas, thyroid adenomas ((NTP, 1995))
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (7 pp, 310 K)
No PBPK model of tert-butyl alcohol in mice is available; therefore route to route extrapolation (oral to inhalation) cannot be performed at this time.
Chemical Documents
Aug 2021: IRIS Toxicological Review of Tert-Butyl Alcohol (Tert-Butanol or TBA) (Final Report) (Report)
Jun 2017: IRIS Toxicological Review of Tert-Butyl Alcohol (TBA) (External Review Draft, 2017) (Report)
May 2016: IRIS Toxicological Review of Tert-Butyl Alcohol (TBA) (Public Comment Draft, 2016) (Report)
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Related Links
Critical Effects
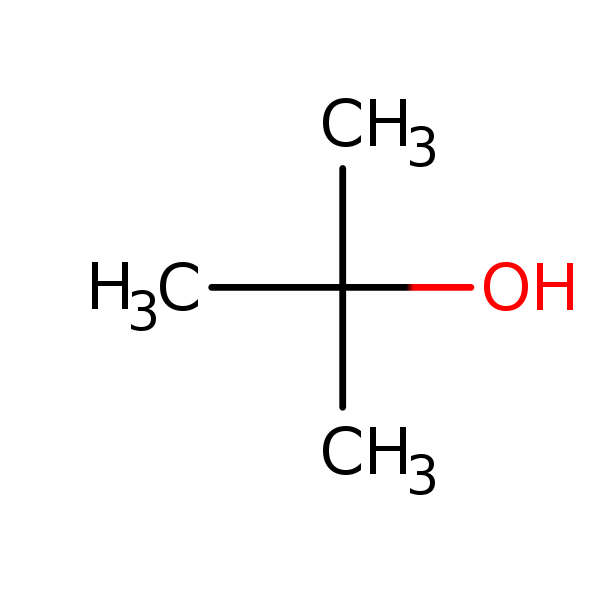
Chemical Structure
Synonyms
- NCI-C55367
- TBA
- arconol
- t-butanol
- t-butyl alcohol
- t-butyl hydroxide
- tBA
- tert-butanol
- tert-butyl alcohol
- tert-butylalcohol
- tertiary carbinol
- trimethyl carbinol
- trimethyl methanol
- trimethylcarbinol
- trimethylmethanol
- 1,1-dimethylethanol
- 2 methylpropan-2-ol
- 2 metilpropan-2-ol
- 2-Propanol
- 2-methyl-
- 2-methyl-2-propanol
- 2-methylpropan-2-ol
- 2-methylpropane-2-ol