Acetaldehyde
CASRN 75-07-0 | DTXSID5039224
- IRIS Summary (PDF) (20 pp, 147 K)
On this page:
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (20 pp, 147 K) Last Updated:
Not assessed under the IRIS Program.
Reference Concentration for Inhalation Exposure (RfC) (PDF) (20 pp, 147 K) Last Updated: 10/01/1991
| System | RfC (mg/m3) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Nervous, Respiratory | 9 x 10 -3 | Degeneration of olfactory epithelium |
NOAEL
(HEC):
8.7
mg/m3 |
1000 | Low |
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(20 pp, 147 K)
Last Updated: 06/30/1988
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| B2 (Probable human carcinogen - based on sufficient evidence of carcinogenicity in animals) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 1986) |
- Based on increased incidence of nasal tumors in male and female rats and laryngeal tumors in male and female hamsters after inhalation exposure.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (20 pp, 147 K)
Not Assessed under the IRIS Program.
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (20 pp, 147 K)
Inhalation Unit Risk:
2.2
x 10-6
per µg/m3
Extrapolation Method: Linearized multistage-variable exposure input form (extra risk)
Tumor site(s): Respiratory
Tumor type(s): Nasal squamous cell carcinoma or adenocarcinoma (Woutersen and Appleman, 1984)
Chemical Documents
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Critical Effects
Tumor Sites
Chemical Structure
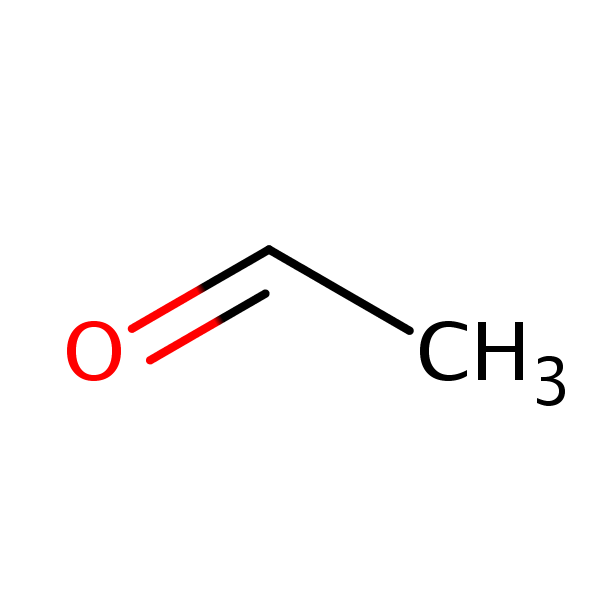
Synonyms
- Acetaldehyd
- Acetaldehyde
- Acetic aldehyde
- Acetylaldehyde
- Aldehyde acetique
- Aldeide acetica
- Ethanal
- Ethyl aldehyde
- NCI-C56326
- Octowy aldehyd
- RCRA Waste Number u001
- UN 1089
- 75-07-0