Parathion
CASRN 56-38-2 | DTXSID7021100
- IRIS Summary (PDF) (10 pp, 100 K)
- Status: EPA announced in a 2004 Federal Register Notice that chemicals used as pesticides would not be re-assessed by the IRIS Program. This entry in the IRIS database is preserved at the request of EPA program and regional offices. Additional toxicological information may be found under "Other EPA Information."
On this page:
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (10 pp, 100 K) Last Updated:
Not assessed under the IRIS Program.
Reference Concentration for Inhalation Exposure (RfC) (PDF) (10 pp, 100 K)
Not assessed under the IRIS Program.
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(10 pp, 100 K)
Last Updated: 08/22/1988
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| C (Possible human carcinogen) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 1986) |
- Increased adrenal cortical tumors in female and male Osborne-Mendel rats and positive trends for thyroid follicular adenomas and pancreatic islet-cell carcinomas in male rats in one study.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (10 pp, 100 K)
Not assessed under the IRIS Program.
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (10 pp, 100 K)
Not assessed under the IRIS Program.
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Related Links
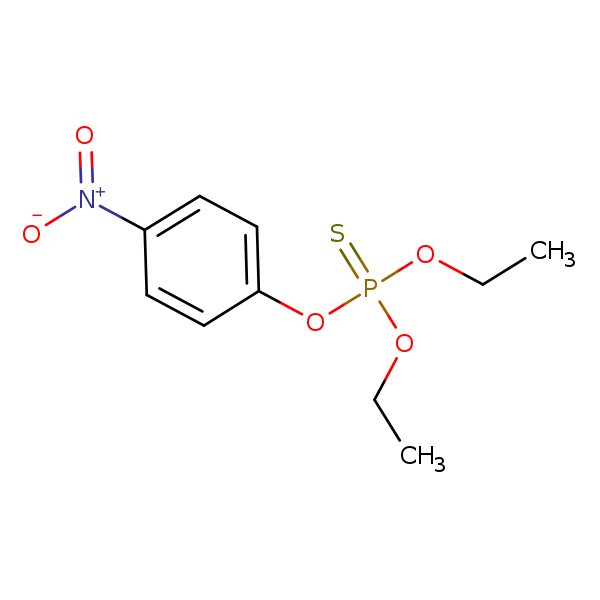
Chemical Structure
Synonyms
- AAT
- AATP
- AC 3422
- ACC 3422
- AI3-15108
- Alkron
- Alleron
- American cyanamid 3422
- Aphamite
- Aralo
- B 404
- Bay E-605
- Bayer E-605
- Bladan
- Bladan f
- Caswell No. 637
- Compound 3422
- Corothion
- Corthion
- Corthione
- DNTP
- DPP
- Danthion
- Diethyl 4-nitrophenyl phosphorothionate
- Diethyl p-nitrophenyl phosphorothionate
- Diethyl p-nitrophenyl thionophosphate
- Diethyl p-nitrophenyl thiophosphate
- Diethyl para-nitrophenol thiophosphate
- Diethyl parathion
- Diethyl-p-nitrophenyl monothiophosphate
- Diethylparathion
- Dietil tiofosfato de p-nitrofenila [portuguese]
- Drexel parathion 8e
- E 605
- E 605 f
- E 605 forte
- ENT 15,108
- EPA Pesticide Chemical Code 057501
- Ecatox
- Ekatin wf wf ulv
- Ekatox
- Ethlon
- Ethyl parathion
- Etilon
- Etylparation [Czech]
- Folidol
- Folidol E
- Folidol E605
- Folidol Oil
- Folidol e e 605
- Fosferno
- Fosfex
- Fosfive
- Fosova
- Fostern
- Fostox
- Gearphos
- Genithion
- HSDB 197
- Kolphos
- Kypthion
- Lethalaire g-54
- Lirothion
- Murfos
- NA 2783
- NCI-C00226
- Niran
- Niran e-4
- Nitrostigmin (German)
- Nitrostigmine
- Nitrostygmine
- Niuif 100
- Nourithion
- OMS 19
- Oleofos 20
- Oleoparaphene
- Oleoparathion
- Orthophos
- PAC
- Pacol
- Panthion
- Paradust
- Paraflow
- Paramar
- Paramar 50
- Paraphos
- Paraspray
- Parathene
- Parathion
- Parathion mixture, dry
- Parathion mixture, liquid
- Parathion, liquid
- Parathion-acetyl [German]
- Parathion-aethyl [German]
- Parathion-ethyl
- Parawet
- Penncap e
- Penphos
- Pestox plus
- Pethion
- Phenol, p-nitro-, o-ester with o,o-diethylphosphorothioate
- Phoskil
- Phosphenol
- Phosphorothioic acid, o,o-diethyl o-(4-nitrophenyl) ester
- Phosphorothioic acid, o,o-diethyl o-(p-nitrophenyl) ester
- Phosphostigmine
- RB
- RCRA Waste Number P089
- Rhodiasol
- Rhodiatox
- Rhodiatrox
- SNP
- Selephos
- Sixty-three special e.c. insecticide
- Soprathion
- Stabilized ethyl parathion
- Stathion
- Strathion
- Sulphos
- Super rodiatox
- T-47
- Thiofos
- Thiomex
- Thiophos
- Thiophos 3422
- Thiophosphate de o,o-diethyle et de o-(4-nitrophenyle) [french]
- Tiofos
- Tox 47
- Vapophor
- Vapophos
- Vitrex
- o,o-Diaethyl-o-(4-nitro-phenyl)-monothiophosphat [german]
- o,o-Diethyl o-(4-nitrophenyl) phosphorothioate (9ci)
- o,o-Diethyl o-(p-nitrophenyl) phosphorothioate
- o,o-Diethyl o-(p-nitrophenyl) phosphorothioate
- o,o-Diethyl o-4-nitrophenyl phosphorothioate
- o,o-Diethyl o-4-nitrophenyl thiophosphate
- o,o-Diethyl o-p-nitrophenyl thiophosphate
- o,o-Diethyl o-p-nitrophenyl thiophosphate
- o,o-Diethyl o-p-nitrophenylphosphorothioate
- o,o-Diethyl-o,p-nitrophenyl phosphorothioate
- o,o-Diethyl-o-(4-nitro-fenil)-monothiofosfaat [dutch]
- o,o-Diethyl-o-(4-nitrophenyl) phosphorothioate
- o,o-Diethyl-o-(p-nitrophenyl)thionophosphate
- o,o-Diethyl-o-p-nitrofenylester kyseliny thiofosforecne [czech]
- o,o-Dietil-o-(4-nitro-fenil)-monotiofosfato [italian]
- o,o-Dietyl-o-p-nitrofenyltiofosfat [czech]
- 56-38-2
