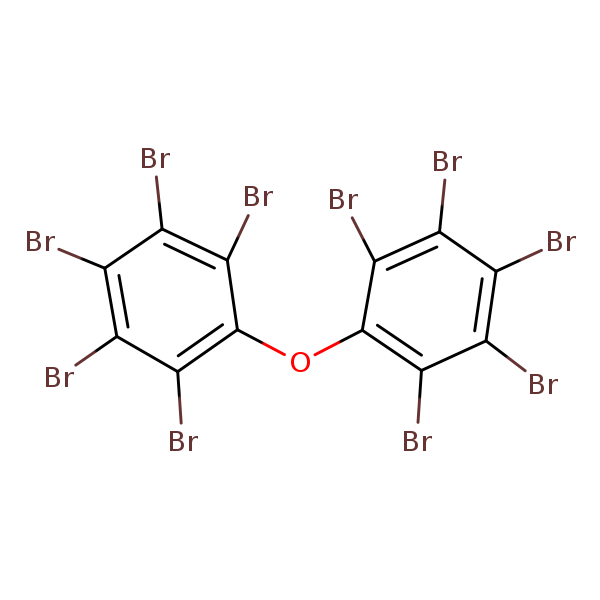
2,2',3,3',4,4',5,5',6,6'-Decabromodiphenyl ether (BDE-209)
CASRN 1163-19-5 | DTXSID9020376
- Toxicological Review (PDF) (126 pp, 820 K)
- IRIS Summary (PDF) (25 pp, 232 K)
On this page:
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (25 pp, 232 K) Last Updated: 06/30/2008
| System | RfD (mg/kg-day) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Nervous | 7 x 10 -3 | Neurobehavioral effects |
NOAEL
:
2.22
mg/kg |
300 | Low |
Reference Concentration for Inhalation Exposure (RfC) (PDF) (25 pp, 232 K) Last Updated: 06/30/2008
Information reviewed but value not estimated.
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(25 pp, 232 K)
Last Updated: 06/30/2008
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| Suggestive evidence of carcinogenic potential | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005) |
- Under the Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005a), the database for decabromodiphenyl ether provides suggestive evidence of carcinogenic potential.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (25 pp, 232 K)
Oral Slope Factor:
7
x 10-4
per mg/kg-day
Drinking Water Unit Risk:
2
x 10-8
per µg/L
Extrapolation Method: Multistage model with linear extrapolation from the point of departure (LED12).
Tumor site(s): Hepatic
Tumor type(s): Liver neoplastic nodules or carcinoma (combined) (NTP (1986))
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (25 pp, 232 K)
Not Assessed under the IRIS Program.
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Related Links
Critical Effects
Tumor Sites
Chemical Structure
Synonyms
- BDE-209
- Benzene, 1,1'-oxybis(2,3,4,5,6-pentabromo-
- DBDPE
- DBDPO
- DecaBDE
- Decabromobiphenyl ether
- Decabromobiphenyl oxide
- Decabromodiphenyl ether
- Decabromodiphenyl oxide
- Decabromophenyl ether
- Ether, bis(pentabromophenyl)
- Ether, decabromodiphenyl
- Pentabromophenyl ether
- 1163-19-5
- 2,2',3,3',4,4',5,5',6,6'-Decabromodiphenyl ether (BDE-209)


