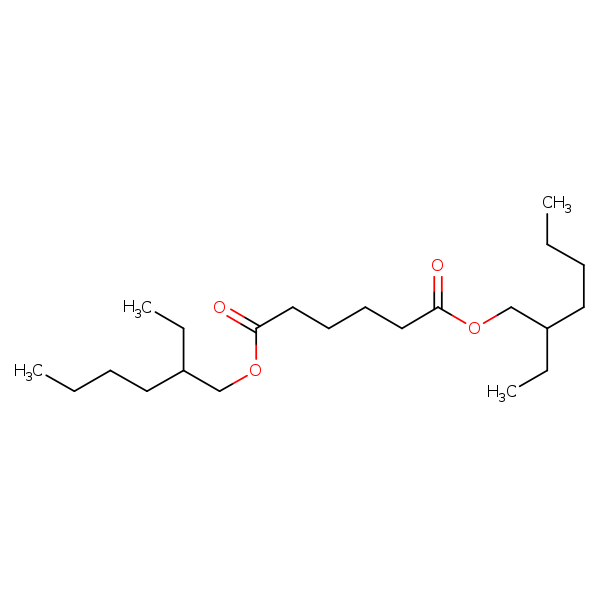
Di(2-ethylhexyl)adipate
CASRN 103-23-1 | DTXSID0020606
- IRIS Summary (PDF) (17 pp, 135 K)
On this page:
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (17 pp, 135 K) Last Updated: 07/01/1992
| System | RfD (mg/kg-day) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Developmental, Hepatic, Urinary, Musculoskeletal, Other | 6 x 10 -1 | Changes in body weight and liver weight increased liver weight of male and female parents reduced ossification and slightly dilated ureters in fetuses reduced offspring weight gain, total litter weight, and litter size |
NOAEL
:
1.70
x 102
mg/kg-day |
300 | Medium |
Reference Concentration for Inhalation Exposure (RfC) (PDF) (17 pp, 135 K)
Not assessed under the IRIS Program.
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(17 pp, 135 K)
Last Updated: 08/01/1991
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| C (Possible human carcinogen) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 1986) |
- Based on an absence of human data and increased incidence of liver tumors in female mice. Except for a positive dominant lethal assay, there was no evidence of genotoxicity; this compound does, however, exhibit structural relationships to other nongenotoxic compounds classified as probable and possible human carcinogens.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (17 pp, 135 K)
Oral Slope Factor:
1.2
x 10-3
per mg/kg-day
Drinking Water Unit Risk:
3.4
x 10-8
per µg/L
Extrapolation Method: Linearized multistage procedure, extra risk
Tumor site(s): Hepatic
Tumor type(s): Combined hepatocellular adenomas and carcinomas (NTP, 1982)
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (17 pp, 135 K)
Not Assessed under the IRIS Program.
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Related Links
Critical Effects
Tumor Sites
Chemical Structure
Synonyms
- Adipic acid, bis(2-ethylhexyl) ester
- Adipol 2EH
- BEHA
- Bisoflex DOA
- DEHA
- DOA
- Di(2-ethylhexyl)adipate
- Di-2-ethylhexyl adipate
- Dioctyl adipate
- Effemoll DOA
- Effomoll DOA
- Ergoplast ADDO
- Flexol A 26
- Flexol Plasticizer A-26
- Flexol plasticizer 10-A
- Hexanedioic acid, bis(2-ethylhexyl) ester (9CI)
- Hexanedioic acid, dioctyl ester
- Kemester 5652
- Kodaflex DOA
- Mollan S
- Monoplex DOA
- NCI-C54386
- Octyl adipate
- PX-238
- Plastomoll DOA
- Reomol DOA
- Rucoflex Plasticizer DOA
- Sicol 250
- Staflex DOA
- Truflex DOA
- Uniflex DOA
- Vestinol OA
- Wickenol 158
- Witamol 320
- bis(2-Ethylhexyl) adipate
- bis-(2-Ethylhexyl)ester kyseliny adipove [Czech]
- 103-23-1