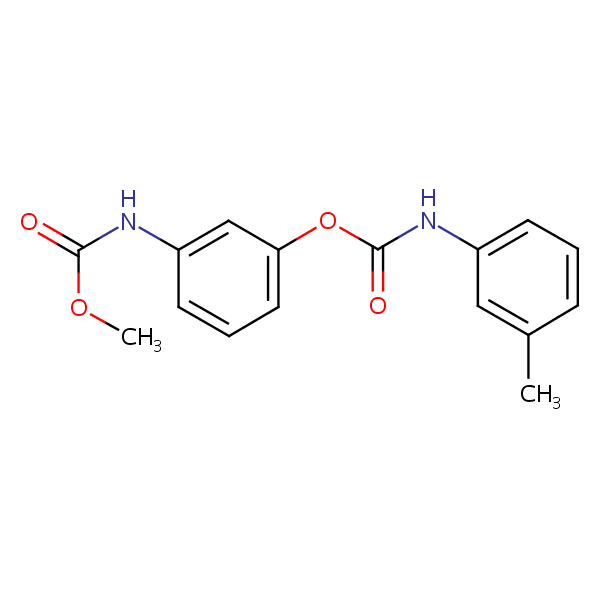
Phenmedipham
CASRN 13684-63-4 | DTXSID1024255
- Archived IRIS Summary (PDF) (8 pp, 100 K)
- Status: EPA announced in a 2004 Federal Register Notice that chemicals used as pesticides would not be re-assessed by the IRIS Program. This entry in the IRIS database is preserved at the request of EPA program and regional offices. Additional toxicological information may be found under "Other EPA Information."
Alert
Notice - This webpage has been Archived
Archive Disclaimer - This site contains archived material.
This web page has been archived and is maintained for reference purposes only. Persons with disabilities having difficulty accessing archived materials may contact the IRIS Hotline for assistance. Please use the contact us form if you need immediate assistance.
This web page has been archived and is maintained for reference purposes only. Persons with disabilities having difficulty accessing archived materials may contact the IRIS Hotline for assistance. Please use the contact us form if you need immediate assistance.