Mercuric chloride (HgCl2)
CASRN 7487-94-7 | DTXSID5020811
- IRIS Summary (PDF) (21 pp, 156 K)
On this page:
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (21 pp, 156 K) Last Updated: 05/01/1995
| System | RfD (mg/kg-day) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Immune, Urinary | 3 x 10 -4 | Autoimmune effects (autoimmune glomerulonephritis) |
LOAEL
:
3.17
x 10-1
mg/kg-day |
1000 | High |
Reference Concentration for Inhalation Exposure (RfC) (PDF) (21 pp, 156 K)
Not assessed under the IRIS Program.
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(21 pp, 156 K)
Last Updated: 05/01/1995
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| C (Possible human carcinogen) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 1986) |
- Based on the absence of data in humans and limited evidence of carcinogenicity in rats and mice. Focal papillary hyperplasia and squamous cell papillomas in the forestomach as well as thyroid follicular cell adenomas and carcinomas were observed in male rats gavaged with mercuric chloride for 2 years. The relevance of the forestomach papillomas to assessment of cancer in humans is questionable because no evidence indicated that the papillomas progressed to malignancy. The relevance of the increase in thyroid tumors has also been questioned because these tumors are generally considered to be secondary to hyperplasia; this effect was not observed in the high-dose males. It should also be noted that the authors considered the doses used in the study to exceed the MTD for male rats. In the same study, evidence for increases in squamous cell papillomas in the forestomach of female rats was equivocal. In a second study, equivocal evidence for renal adenomas and adenocarcinomas was observed in male mice; there was a significant positive trend. This tumor type is rare in mice, and the increase in incidence was statistically significant when compared with historic controls. Two other nonpositive lifetime rodent studies were considered inadequate. Mercuric chloride showed mixed results in a number of genotoxicity assays.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (21 pp, 156 K)
Not assessed under the IRIS Program.
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (21 pp, 156 K)
Not assessed under the IRIS Program.
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Related Links
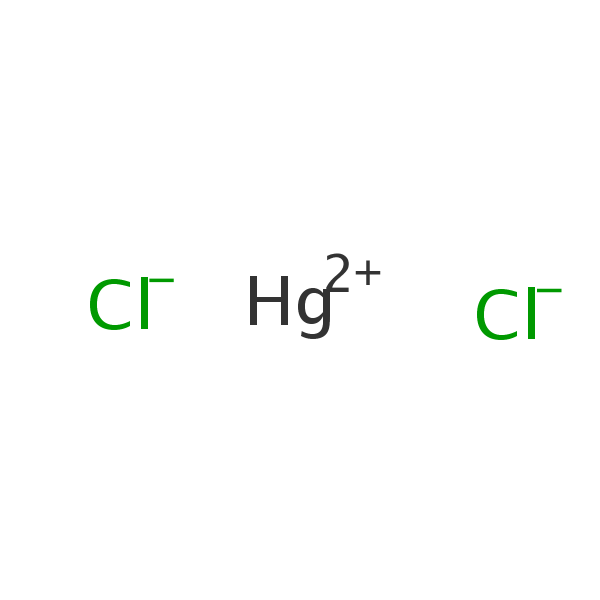
Chemical Structure
Synonyms
- Bichloride of mercury
- Bichlorure de mercure [French]
- Caswell No. 544
- Chlorid rtutnaty [Czech]
- Chlorure de mercure ii [French]
- Chlorure mercurique [French]
- Cloruro di mercurio [Italian]
- Cloruro mercurico [Spanish]
- Corrosive mercury chloride
- Corrosive sublimate
- Dichloromercury
- EPA Pesticide Chemical Code 052001
- HGCL2
- Mercuric bichloride
- Mercuric chloride
- Mercuric chloride(HgCl2)
- Mercury bichloride
- Mercury chloride (HGCL2)
- Mercury dichloride
- Mercury perchloride
- Mercury(ii) chloride
- NCI-C60173
- NSC 353255
- Quecksilber chlorid [German]
- Sublimat [Czech]
- Sulema [Russian]
- 7487-94-7