Hexachloroethane
CASRN 67-72-1 | DTXSID7020689
- Toxicological Review (PDF) (200 pp, 1.5 MB, about PDF)
- IRIS Summary (PDF) (34 pp, 300.8 KB, about PDF)
IRIS Toxicological Review of Hexachloroethane (Interagency Science Consultation Draft)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
EPA is releasing the draft report, Toxicological Review of Hexachloroethane, that was distributed to Federal agencies and White House Offices for comment during the Science Discussion step of the IRIS Assessment Development Process. Comments received from other Federal agencies and White House Offices are provided below with external peer review panel comments.Background
The draft Toxicological Review of hexachloroethane provides scientific support and rationale for the hazard and dose-response assessment pertaining to chronic exposure to hexachloroethane. Hexachloroethane (C2Cl6) is a colorless, nonflammable, crystalline solid primarily used in the military for pyrotechnic devices. Hexachloroethane has been used as a polymer additive, moth repellant, plasticizer for cellulose esters, insecticide solvent, and for refining aluminum alloys. Hexachloroethane is not commercially distributed in the United States. Exposure to hexachloroethane is primarily occupational, via inhalation and dermal exposure during hexachloroethane manufacturing and metal refining. Exposure may occur in military or civilian personnel working with pyrotechnic devices containing hexachloroethane and via ingestion of contaminated drinking water or fish. Hexachloroethane was identified in Superfund sites.This assessment contains the derivation of a chronic oral reference dose (RfD), chronic inhalation reference concentration (RfC), and an oral cancer slope factor.
| Date | Description |
|---|---|
| 1987 | The oral RfD and cancer assessment for hexachloroethane was posted to the IRIS database. |
| Apr 2010 | EPA initiated the interagency science consultation on the draft Toxicological Review of Hexachloroethane. |
| May 2010 | EPA released the draft Toxicological Review and federal agency comments from the Interagency Science consultation. Additionally, EPA released the External Review Draft of Hexachloroethane for public review and comment. |
Status
Following external peer review the draft report will be revised taking into consideration external peer review and public comments, will undergo a final EPA internal review and a review by a science discussion with other federal agencies and White House offices, and then will finally be posted to the IRIS Web site.Download(s)
This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
- Toxicological Review of Hexachloroethane: in Support of Summary Information on the Integrated Risk Information System (IRIS) (Interagency Science Consultation Draft) (PDF) (209 pp, 1.3 MB, about PDF)
- Charge to External Reviewers (Interagency Science Consultation Draft) (PDF) (3 pp, 31.2 KB, about PDF)
- DOD Comments of the Review of Hexachloroethane (Mar 2010) (PDF) (1 pp, 27.3 KB, about PDF)
- OMB Comments of the Review of Hexachloroethane (Apr 2010) (PDF) (4 pp, 38.1 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Tumor Sites
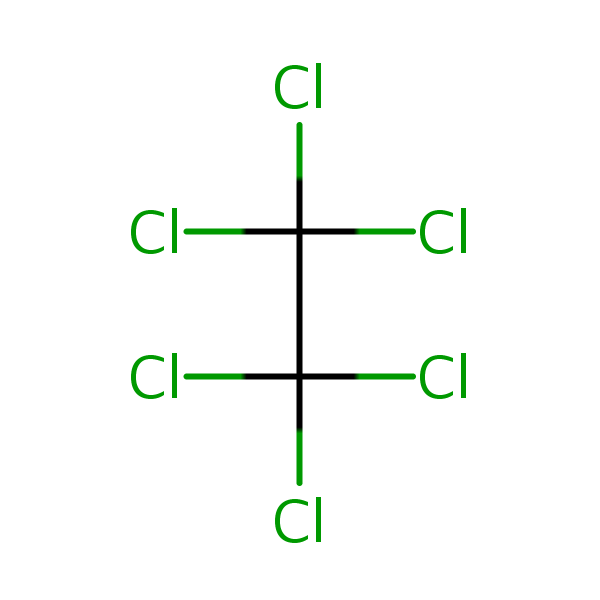
Chemical Structure for
Hexachloroethane
Synonyms
- Avlothane
- Carbon hexachloride
- Distokal
- Distopan
- Distopin
- Egitol
- Ethane hexachloride
- Ethylene hexachloride
- Falkitol
- Fasciolin
- Hexachlor-aethan
- Hexachloroethane
- Hexachloroethylene
- Mottenhexe
- NA 9037
- NCI-C04604
- Perchloroethane
- Phenohep
- RCRA Waste Number U131
- 1,1,1,2,2,2-Hexachloroethane
- 67-72-1