Acrylonitrile
CASRN 107-13-1 | DTXSID5020029
- IRIS Summary (PDF) (23 pp, 158.2 KB, about PDF)
- Status: Development of the acrylonitrile (re)assessment has been discontinued.
IRIS Toxicological Review of Acrylonitrile (External Review Draft)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
[UPDATE] New Schedule for IRIS Acrylonitrile Assessment
In May 2012, EPA developed a new schedule for completing the IRIS acrylonitrile assessment. Acrylonitrile is primarily used in the manufacture of acrylic and modacrylic fibers, plastics, and nitrile rubbers. It is also used as an intermediate in the production of other chemicals.EPA has decided to make further revisions to the acrylonitrile assessment to more fully address scientific issues in the assessment. The revised draft acrylonitrile assessment will be released for public comment and rigorous, independent expert peer review. EPA will seek to have the peer review performed by the new Chemical Assessment Advisory Committee that is being formed under the auspices of the Science Advisory Board.
Background
The draft Toxicological Review of Acrylonitrile provides scientific support and rationale for the hazard and dose-response assessment pertaining to chronic exposure to acrylonitrile. Acrylonitrile is widely used in the production of acrylic fibers, plastics (acrylonitrile butadiene styrene and acrylonitrile-styrene resins), and nitrile rubbers, and as an intermediate in the production of other chemicals.| Date | Description |
|---|---|
| Sep 1987 | The cancer assessment for acrylonitrile was posted to the IRIS database. |
| Nov 1991 | The inhalation RfC for acrylonitrile was posted to the IRIS database. |
| Mar 2010 | EPA hosted an interagency science consultation on the review of the draft Toxicological Review of Acrylonitrile. |
| Jun 2010 | Following a report from the National Toxicology Program, EPA placed the draft Acrylonitrile Toxicological Review on hold. [See June 15, 2010 SAB press release] |
| Jun 2011 | EPA hosted a second interagency science consultation on the review of the selected sections and highlights to the external peer review draft of the Toxicological Review of Acrylonitrile. |
| Jun 2011 | EPA released the external review draft of the acrylonitrile assessment for public review and comment. [Federal Register Notice Jun 30, 2011] |
| Aug 2011 | EPA announced a 30-day extension of the public comment period for the document, IRIS Toxicological Review of Acrylonitrile (External Review Draft) (EPA/635/R-08/013A). The comment period closed on September 28, 2011. [Federal Register Notice Aug 31, 2011] |
| Sep 2011 | EPA announced the availability of additional documentation for the acrylonitrile physiologically based pharmacokinetic (PBPK) model (Zip file under downloads). This documentation supplements Appendix C of the IRIS Toxicological Review of Acrylonitrile (External Review Draft). |
Status
This draft has been archived.Additional Information
Comments on the assessment may be submitted and reviewed using the e-Government Regulations.gov Web site. From the site, select “Environmental Protection Agency” and the keyword “EPA-HQ-ORD-2009-0204 (for the docket ID) to comment on this report.Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- IRIS Toxicological Review of Acrylontrile (External Review Draft) (PDF) (651 pp, 3.7 MB, about PDF)
- Charge to External Peer Reviewers (PDF) (4 pp, 75.2 KB, about PDF)
- Acrylonitrile physiologically based pharmacokinetic (PBPK) modeling code (ZIP) (584.0 KB, about ZIP)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
Federal Register Notices
Related Links
Critical Effect Systems
Tumor Sites
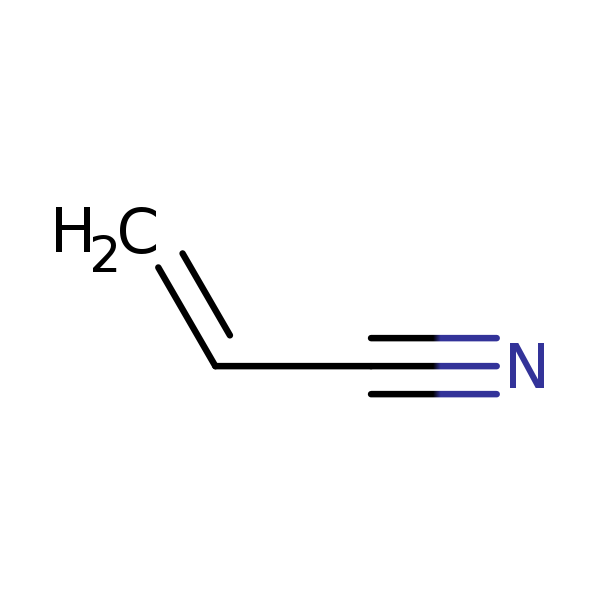
Chemical Structure for
Acrylonitrile
Synonyms
- Acritet
- Acrylnitril
- Acrylon
- Acrylonitrile
- Acrylonitrile monomer
- Akrylonitryl
- Carbacryl
- Cianuro di vinile
- Cyanoethylene
- Cyanure de vinyle
- ENT 54
- Fumigrain
- Miller's Fumigrain
- Nitrile acrilico
- Nitrile acrylique
- Propenenitrile
- RCRA Waste Number u009
- TL 314
- UN 1093
- VCN
- Ventox
- Vinyl cyanide
- 107-13-1
- 2-Propenenitrile