Formaldehyde
CASRN 50-00-0 | DTXSID7020637
- Toxicological Review (PDF) (927 pp, 16.3 MB, about PDF)
- IRIS Executive Summary (PDF) (10 pp, 450.2 KB, about PDF)
IRIS Toxicological Review of Formaldehyde-Inhalation (External Review Draft, 2022)
On this page:
Alert
Notice
The National Academies of Sciences, Engineering, and Medicine (NASEM) announced the release their final report, Review of EPA's 2022 Draft Formaldehyde Assessment, which provides recommendations for improving EPA's draft IRIS assessment. [NASEM Press Release Notice: Aug 9, 2023]
Overview
In April 2022, EPA publicly released the draft formaldehyde assessment for review and comment. The IRIS Toxicological Review of Formaldehyde-Inhalation provides the scientific support and rationale for the hazard and dose-response assessment of inhalation exposure to formaldehyde. An independent external scientific peer review managed by the National Academies of Sciences, Engineering, and Medicine (NASEM), under contract with EPA was completed in August 2023.Background
Formaldehyde is present in a variety of products including plywood adhesives, abrasive materials, insulation, pesticides, and embalming fluids. Major sources of anthropogenic emissions include household furnishings and building materials, motor vehicle exhaust, manufacturing plants that produce or use formaldehyde or substances that contain it (e.g., glues), and tobacco smoke.| Date | Description |
|---|---|
| May 2011 | The National Academy of Sciences (NAS) Board on Environmental Studies and Toxicology (BEST) released the NAS Review of the Environmental Protection Agency's Draft IRIS Assessment of Formaldehyde (Final Report). |
| Apr 2014 | EPA convened a state-of-the-science workshop on formaldehyde on April 30 and May 1, 2014 in Arlington, VA. This workshop focused on 3 themes, see the web site for specific details. |
| Jun 2018 | The IRIS Assessment of Formaldehyde (Inhalation) Agency Review draft (completed in 2017) was formally suspended. |
| Mar 2021 | EPA updated the list of priority assessments developed by IRIS. EPA unsuspends the IRIS Toxicological Review of Formaldehyde (Inhalation). |
| Dec 2021 | EPA initiated an interagency science consultation on the draft Toxicological Review of Formaldehyde (Inhalation). |
| Apr 2022 | EPA released the draft Toxicological Review of Formaldehyde-Inhalation (External Review Draft) for public comment and subsequent external peer review by the National Academies of Sciences, Engineering, and Medicine (NASEM). Also released was the EPA-led Interagency Science Consultation draft with interagency comments. The draft report was open for a 60-day public comment period and closed on June 13, 2022. [FR Notice: Apr 14, 2022] |
| Aug 2023 | The National Academies of Sciences, Engineering, and Medicine (NASEM) released their consensus study report, Review of EPA's 2022 Draft Formaldehyde Assessment, which provides recommendations to the U.S.EPA. |
Status
In April 2022, the assessment was released for a 60-day public comment period. An independent, external scientific peer review managed by the National Academies of Sciences, Engineering, and Medicine (NASEM), under contract with EPA, was completed in August 2023.Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- Toxicological Review of Formaldehyde-Inhalation (External Review Draft) (PDF) (789 pp, 14.1 MB, about PDF)
- Toxicological Review of Formaldehyde-Inhalation, Supplemental Information (External Review Draft) (PDF) (1058 pp, 15.9 MB, about PDF)
- Assessment Overview for the Toxicological Review of Formaldehyde-Inhalation (PDF) (193 pp, 4.5 MB, about PDF)
- Final Charge Questions to Reviewers (June 2022) (PDF) (10 pp, 278.3 KB, about PDF)
- NASEM Review of EPA's 2022 Draft Formaldehyde Assessment (Final Report)
- IRIS TOXICOLOGICAL REVIEW OF FORMALDEHYDE (INHALATION) (FINAL REPORT, 2024) (PDF) (927 pp, 159.3 MB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
Federal Register Notices
Docket
Comments on the assessment may be submitted and reviewed using the Docket ID EPA-HQ-ORD-2010-0396Related Links
Critical Effect Systems
Tumor Sites
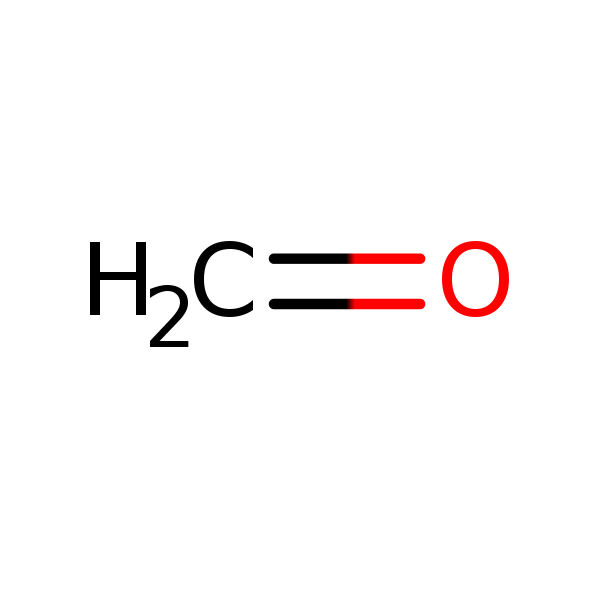
Chemical Structure for
Formaldehyde
Synonyms
- Aldehyd mravenci (Czech)
- Aldehyde formique (French)
- Aldeide formica (Italian)
- BFV
- FA
- Formaldehyd (Czech, Polish)
- Formaldehyde
- Formaldehyde solution (DOT)
- Formalin
- Formalith
- Formic aldehyde
- Formol
- Fyde
- Hoch
- Ivalon
- Karsan
- Lysoform
- Methanal
- Methyl aldehyde
- Methylene glycol
- Methylene oxide
- Morbicid
- NCI-C02799
- Oplossingen (Dutch)
- Oxomethane
- Oxymethylene
- Paraform
- Polyoxymethylene glycols
- RCRA Waste Number U122
- Superlysoform
- UN 1198 (DOT)
- UN 2209 (DOT)
- 50-00-0