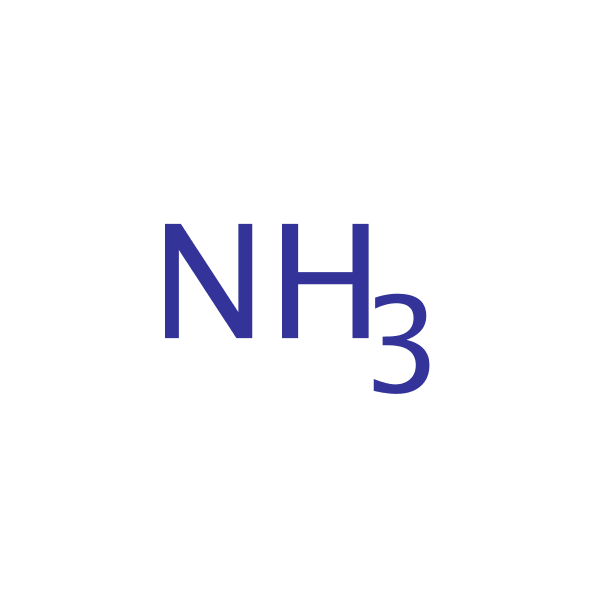
Ammonia
CASRN 7664-41-7 | DTXSID0023872
IRIS Assessment Plan for Ammonia and Ammonium Salts Noncancer Oral (Scoping and Problem Formulation Materials, Suspended)
On this page:
Alert
Notice
[UPDATE] EPA suspended the Ammonia and Ammonium Salts (noncancer oral exposure) assessment in fiscal year 2019. This assessment may be restarted as Agency priorities change. For more information, visit the IRIS Program Outlook (Apr 2019).
Overview
In April 2018, EPA released the draft IRIS Assessment Plan for Ammonia and Ammonium Salts Noncancer Oral. An IRIS Assessment Plan (IAP) communicates to the public the plan for assessing each individual chemical and includes summary information on the IRIS Program’s scoping and initial problem formulation; objectives and specific aims for the assessment; the PECO (Populations, Exposures, Comparators, and Outcomes) criteria that outlines the evidence considered most pertinent to the assessment; and identification of key areas of scientific complexity. The PECO provides the framework for developing literature search strategies and inclusion/exclusion criteria, particularly with respect to evidence stream (i.e., human, animal, mechanistic), exposure measures and outcome measures.Background
Ammonia occurs naturally in air, soil, and water. Ammonia is also produced by humans and other animals as part of normal biological processes. Ammonia is used as an agricultural fertilizer and in many cleaning products. Exposure to ammonia occurs primarily through breathing air containing ammonia gas, and may also occur via diet, drinking water, or direct skin contact.| Date | Description |
|---|---|
| Apr 2019 | EPA suspended the Ammonia and Ammonium Salts (noncancer oral exposure) assessment in fiscal year 2019. This assessment may be restarted as Agency priorities change. |
| Apr 2018 | EPA released the IRIS Assessment Plan for Ammonia and Ammonium Salts (noncancer oral exposure) for a 30-day public comment period.[Federal Register Notice Apr 16, 2018] |
| Sep 2016 | EPA released the final IRIS Toxicological Review of Ammonia (noncancer Inhalation) to the IRIS database. |
Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- IRIS Assessment Plan for Ammonia and Ammonium Salts: Noncancer Assessment for Oral Exposure (Scoping and Problem Formulation Materials) (PDF) (32 pp, 788.1 KB, about PDF)
- Docket for EPA-HQ-ORD-2018-0132
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
HERO Project
Federal Register Notices
Related Links
Critical Effect Systems
Chemical Structure for
Ammonia
Synonyms
- Am-Fol
- Ammonia
- Ammonia
- Ammonia gas
- Ammonia solution, strong
- Ammoniac [French]
- Ammoniaca [Italian]
- Ammoniak [German]
- Amoniaco [Spanish]
- Amoniak [Polish]
- Anhydrous ammonia
- Aromatic ammonia, vaporole
- Caswell No. 041
- EPA Pesticide Chemical Code 005302
- HSDB 162
- Nitro-sil
- R 717
- Spirit of Hartshorn
- UN 1005
- UN 2073
- UN 2672
- 7664-41-7