Ethyl Tertiary Butyl Ether (ETBE)
CASRN 637-92-3 | DTXSID0025604
- Toxicological Review (PDF) (200 pp, 4.1 MB, about PDF)
- IRIS Executive Summary (PDF) (8 pp, 291.0 KB, about PDF)
- Supplemental Information on the IRIS Toxicological Review of Ethyl Tertiary Butyl Ether
IRIS Toxicological Review of Ethyl Tertiary Butyl Ether (ETBE) (Interagency Science Discussion Draft, 2020)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
In August 2021, EPA finalized the IRIS assessment of ethyl tertiary butyl ether (ETBE). The Toxicological Review was reviewed internally by EPA and by other federal agencies and White House Offices after public release in June 2017. Consistent with the May 2009 IRIS assessment development process, all written comments on IRIS assessments submitted by other federal agencies and White House Offices are made publicly available. Accordingly, interagency comments and the interagency science discussion materials provided to other agencies, including interagency review drafts of the IRIS Toxicological Review of Ethyl Tertiary Butyl Ether are posted on this site.Background
ETBE is used as a fuel additive for gasoline to increase octane rating and has been used to meet air pollution reduction goals in the U.S. under the Clean Air Act. The use of ether oxygenates such as ethyl tertiary butyl ether in reformulated gasoline in the United States was effectively eliminated in 2006; however, use and production of these oxygenates has continued in Europe and Asia for the purpose of increasing octane rating in gasoline. ETBE may be used as a substitute to replace the volume and oxygenate properties of methyl tertiary butyl ether, an oxygenate widely used in the United States prior to 2006. The main source of exposure to ETBE in U.S. is from exposure to contaminated groundwater due to leaking underground storage tanks.| Date | Description |
|---|---|
| Aug 2013 | EPA released preliminary assessment materials (e.g., literature searches and associated search strategies, evidence tables, and exposure response arrays) of ETBE for public discussion at the December 2013 IRIS Bimonthly Public Meeting. |
| Dec 2013 | EPA convened public science meeting to discuss preliminary assessment materials. |
| Sep 2014 | EPA submitted the draft assessments of ETBE for Interagency Science Consultation. |
| Sep 2016 | EPA announced the availability of the draft assessment of ETBE for public comment and discussion and released the interagency science consultation draft, EPA response to select comments, and comments from reviewers. [Federal Register Notice Sep 1, 2016] |
| Oct 2016 | EPA convened a public science meeting to discuss the public comment draft of the IRIS assessment of ETBE. |
| Jun 2017 | EPA released the external review draft and charge to reviewers prior to the public peer review meeting.[SAB FR Notice Jun 16, 2017] |
| Jul 2017 | EPA's SAB CAAC convenes to discuss the external review draft of the IRIS assessment. |
| Feb 2019 | EPA's SAB released their review of the draft IRIS assessment. |
| Aug 2020 | EPA submitted the revised interagency science discussion draft for final Agency and Interagency review. |
| Aug 2021 | EPA posted the final report IRIS Toxicological Review of Ethyl Tertiary Butyl Ether (ETBE) and released the interagency science discussion draft and comments to the IRIS database. |
Status
Now that the interagency review draft has been completed and finalized, the ETBE assessment has been loaded into the IRIS Web site and Database.Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- IRIS Toxicological Review of Ethyl Tertiary Butyl Ether (Interagency Science Discussion Draft) (PDF) (191 pp, 4.2 MB, about PDF)
- IRIS Toxicological Review of Ethyl Tertiary Butyl Ether - Supplemental Information (Interagency Science Discussion Draft) (PDF) (177 pp, 3.7 MB, about PDF)
- EPA's Response to Major Science Comments (Interagency Science Discussion Draft) (PDF) (2 pp, 150.4 KB, about PDF)
- 1. CDC/NIOSH Comments on ETBE (PDF) (3 pp, 124.0 KB, about PDF)
- 2. OMB Comments on ETBE (PDF) (1 pp, 118.2 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
- IRIS Toxicological Review of Ethyl Tertiary Butyl Ether (ETBE) (Interagency Science Consultation Draft, 2016)
- IRIS Toxicological Review of Ethyl Tertiary Butyl Ether (ETBE) (Preliminary Assessment Materials, 2013)
- IRIS Toxicological Review of Ethyl Tertiary Butyl Ether (ETBE) (Public Comment Draft, 2016)
- IRIS Toxicological Review of Ethyl Tertiary Butyl Ether (ETBE) (External Review Draft, 2017)
- IRIS Toxicological Review of Ethyl Tertiary Butyl Ether (ETBE) (Final Report)
Related Links
Critical Effect Systems
Tumor Sites
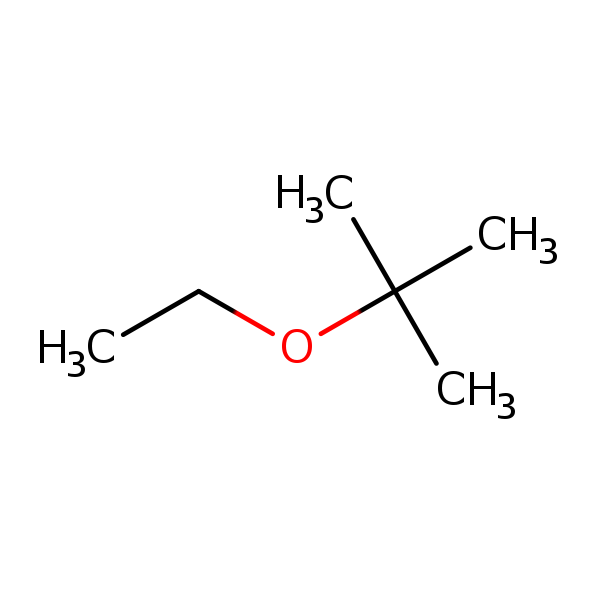
Chemical Structure for
Ethyl Tertiary Butyl Ether (ETBE)
Synonyms
- ETBE
- ethyl 1,1-dimethylethyl ethyl ether
- ethyl tert-butyl ether
- ethyl tert-butyl oxide
- ethylene glycol t-butyl ether
- methyl-2-ethoxypropane
- tert-butyl ethyl ether
- 2-ethoxy-2-methylpropane
- 2-methyl-2-ethoxypropane
- 637-92-3