Ethylene glycol monobutyl ether (EGBE) (2-Butoxyethanol)
CASRN 111-76-2 | DTXSID1024097
- Toxicological Review (PDF) (206 pp, 3.5 MB, about PDF)
- IRIS Summary (PDF) (31 pp, 290.0 KB, about PDF)
An Evaluation of the Human Carcinogenic Potential of Ethylene Glycol Butyl Ether (EGBE)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
This position paper, An Evaluation of the Human Carcinogenic Potential of Ethylene Glycol Butyl Ether, was developed in support of the EPA's evaluation of a petition from the American Chemistry Council requesting to delist EGBE per the Clean Air Act Amendments (CAAA), Title III, section 112(b)(1). The position paper was a key component of the Agency's recent determination to grant this petition. It will also be used in the Agency's IRIS assessment of ethylene glycol butyl ether (EGBE).Background
An NTP (1998; 2000) study has reported some evidence of carcinogenic activity in male B6C3F1 mice based on increased incidence of hemangiosarcomas of the liver, and some evidence of carcinogenic activity in female B6C3F1 mice based on increased incidence of forestomach squamous cell papillomas or carcinomas. EPA completed an IRIS assessment for EGBE in 1999 and concluded that, in accordance with 1996 proposed Guidelines for Carcinogen Risk Assessment (U.S. EPA, 1996), the human carcinogenicity of EGBE cannot be determined, but that suggestive evidence exists from rodent studies. This position paper reviews recent scientific findings and provides an up-to-date evaluation of the mode-of-action (MOA) involved in the origin of these tumors in mice and their human relevance that is consistent with recent EPA draft cancer guidelines (U.S. EPA, 2003). The position paper proposes:- that nonlinear MOAs are associated with tumor development in laboratory animals,
- that aspects of these MOAs were not relevant to humans and,
- that noncancer benchmarks (RfC/RfD values) would be protective of cancer effects.
- Female Mouse Forestomach Tumors - Due to the lack of a comparable organ for storage and long term retention, the exposure concentrations necessary to cause hyperplastic effects and tumors in humans, if attainable, are likely to be much higher than the concentrations necessary to cause forestomach effects in mice, and much higher than the existing EGBE noncancer reference dose (RfD) and concentration (RfC).
- Liver Tumors - Existing evidence supports a hemolysis-dependent, nonlinear MOA for the development of tumors in male mice exposed to EGBE. Given the relatively low sensitivity of humans, including subpopulations such as children, to the hemolytic effects of EGBE, it is reasonable to assume that the EGBE RfC and RfD are sufficient for the prevention of hemolysis and associated tumors in humans.
| Date | Description |
|---|---|
| 1999 | EPA/NCEA finalizes and distributes EGBE assessment online. |
| 2002 | Chemical Manufacturers Association (CMA) presents new information and new studies of EGBE to EPA/NCEA. |
| 2003 | EPA/NCEA reviews the draft position paper internally and externally. |
| 2003 | EPA/NCEA delivers interim final position paper to EPA/OAQPS and EPA/OAQPS promulgates Proposed Rule to delist EGBE. |
| 2004 | CMA and others submit new information and new studies of EGBE to EPA/OAQPS. |
| 2004 | External panel of experts completes review of the interim final position paper in light of new studies. |
| 2004 | EPA/OAQPS promulgates Final Rule to delist EGBE. |
| 2005 | EPA/NCEA finalizes the EGBE Position Paper. |
Download(s)
- AN EVALUATION OF THE HUMAN CARCINOGENIC POTENTIAL OF EHTYLENE GLYCOL BUTYL ETHER (PDF) (92 pp, 480.5 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Related Links
Critical Effect Systems
Chemical Structure for
Ethylene glycol monobutyl ether (EGBE) (2-Butoxyethanol)
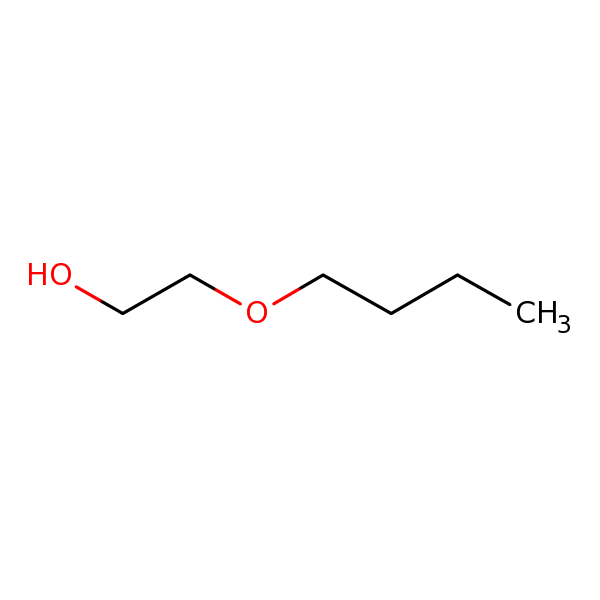
Synonyms
- Bucs
- Butoxyethanol
- Butyl Cellosolve
- Butyl Glycol
- Butyl Oxitol
- CASRN -- 111-76-2
- Dowanol EB
- Ektasolve EB
- Ethylene Glycol N-Butyl
- Ethylene glycol monobutyl ether (EGBE)
- Ethylene glycol monobutyl ether (EGBE) (2-Butoxyethanol)
- Gafcol EB
- Glycol Butyl Ether
- Glycol Ether EB
- Glycol Ether EB Acetate
- Glycol Monobutyl Ether
- Jeffersol EB
- Monobutyl Ether Of Ethylene Glycol
- Monobutyl Glycol Ether
- N-Butoxyethanol
- O-Butyl Ethylene Glycol
- Poly-Solv EB
- 2-Butoxy-1-Ethanol
- 2-Butoxyethanol
- 3-Oxa-1-Heptanol

