Dibutyl phthalate (DBP)
CASRN 84-74-2 | DTXSID2021781
- IRIS Summary (PDF) (11 pp, 104.5 KB, about PDF)
- Status: Development of the dibutyl phthalate (dbp) (re)assessment has been discontinued.
IRIS Toxicological Review of Dibutyl Phthalate (DBP) (External Review Draft) 2006
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
The U.S. EPA has conducted a peer review of the scientific basis supporting the human health hazard and dose-response assessment of dibutyl phthalate that will appear on the Integrated Risk Information System (IRIS) database. Peer review is meant to ensure that science is used credibly and appropriately in derivation of the dose-response assessments and toxicological characterization.Background
Dibutyl phthalate is used as a plasticizer for nitrocellulose, polyvinyl acetate, and polyvinyl chloride; a lubricant for aerosol valves; an antifoaming agent; a skin emollient; and a plasticizer in nail polish, fingernail elongators, and hair spray. Dibutyl phthalate is: 1) classified as a Hazardous Air Pollutant under the 1990 Clean Air Act amendments; 2) included on the Office of Prevention, Pesticides and Toxic Substances 1990 high production volume chemical list; 3) listed as a Toxic Release Inventory chemical; and 4) found at hazardous waste and Superfund sites. The assessment will present reference values for the noncancer effects of dibutyl phthalate (RfD and RfC), where supported by available data, and a cancer assessment. The Toxicological Review will be subject to an internal and external peer review.| Date | Description |
|---|---|
| Jun 2006 | EPA announces an external peer-review workshop to review the external review draft document. |
| Jul 2006 | A panel of external peer reviewers met to discuss their responses to the charge questions. |
| Aug 2006 | EPA announced the release of the consolidated comments of the external peer reviewers. |
Status
This draft has been archived.Additional Information
EPA is hereby providing for public information a draft Toxicological Review, draft IRIS Summary, and charge to external peer reviewers for EPA's health assessment of dibutyl phthalate. These documents are provided for public viewing and comment. Public comments received on the Toxicological Review or IRIS Summary prior to July 14, 2006 were provided to the external peer reviewers for their consideration. Any comments received by July 21, 2006 were considered by EPA in subsequent drafts.Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- IRIS TOXICOLOGICAL REVIEW OF DIBUTYL PHTHALATE (DI-N-BUTYL PHTHALATE) (EXTERNAL REVIEW DRAFT) (PDF) (133 pp, 914.1 KB, about PDF)
- SUMMARY DOCUMENTS FOR DIBUTYL PHTHALATE (DI-N-BUTYL PHTHALATE) (EXTERNAL REVIEW DRAFT) (PDF) (17 pp, 103.5 KB, about PDF)
- Charge to External Reviewers for the IRIS Toxicological Review for Di-n-Butyl Phthalate (PDF) (3 pp, 38.1 KB, about PDF)
- CONSOLIDATED COMMENTS FROM THE EXTERNAL PEER REVIEW (FINAL) (PDF) (21 pp, 114.3 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Federal Register Notices
Related Links
Chemical Structure for
Dibutyl phthalate (DBP)
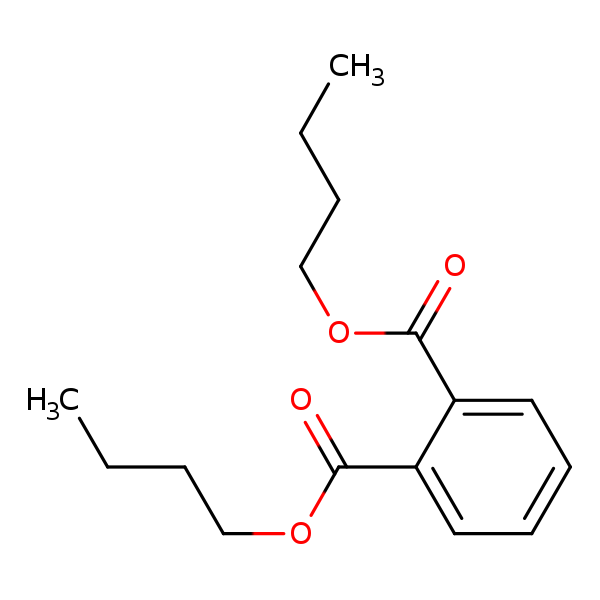
Synonyms
- Benzene-o-dicarboxylic acid di-n-butyl ester
- Butylphthalate
- Celluflex DPB
- DPB
- Di-n-butylphthalate
- Dibutyl 1,2-benzene dicarboxylate
- Dibutyl phthalate
- Dibutyl-o-phthalate
- Elaol
- Ergoplast FDB
- Genoplast B
- Hexaplast M/B
- PX 104
- Palatinol C
- Phthalic acid dibutyl ester
- Polycizer DBP
- RC Plasticizer DBP
- n-Butylphthalate
- o-Benzenedicarboxylic acid, dibutyl ester
- 1,2-Benzenedicarboxylic acid dibutyl ester
- 84-74-2
