2,2,4-Trimethylpentane
CASRN 540-84-1 | DTXSID7024370
- Toxicological Review (PDF) (43 pp, 304.7 KB, about PDF)
- IRIS Summary (PDF) (12 pp, 166.0 KB, about PDF)
IRIS Toxicological Review and Summary Documents For 2,2,4-Trimethylpentane (External Review Draft)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
EPA has conducted a peer review of the scientific basis supporting the human health hazard and dose-response assessment of 2,2,4-trimethylpentane, also called TMP, that will appear on the Integrated Risk Information System (IRIS) database. Peer review is meant to ensure that science is used credibly and appropriately in derivation of the dose-response assessments and toxicological characterization.Background
The draft Toxicological Review of 2,2,4-Trimethylpentane provides scientific information on the effects pertaining to exposure to 2,2,4-trimethylpentane. 2,2,4-Trimethylpentane (TMP) was nominated by OTAQ and OAQPS for evaluation by the IRIS Program, based on its classification as a Title I Clean Air Act Hazardous Air Pollutant and because it is a major component of motor vehicle exhaust. TMP is a hydrocarbon and is also known as isooctane or isobutyltrimethylmethane. TMP is released into the environment through the manufacture, use, and disposal of products associated with the gasoline and petroleum industry. TMP comprises about 10% of unleaded gasoline. Automotive exhaust and automotive evaporative emissions are important sources of atmospheric TMP. Thus, the most probable route of exposure to the general population is by inhalation.The current TMP assessment posted on IRIS (1991) does not include an evaluation of the oral noncancer database or the cancer database, and contains a message for the inhalation RfC that the health effects data for TMP were reviewed by the U.S. EPA RfD/RfC Work Group and were determined to be inadequate for the derivation of an inhalation RfC. An evaluation of the TMP database is not available from other agencies. As a part of the IRIS nomination process for selecting chemicals for assessment, a quick literature review of the database was conducted in 2003. While no new critical studies via the inhalation route were identified, newer mechanistic studies, gavage studies, and toxicokinetic studies were found. This information has been used to update the assessment.
| Date | Description |
|---|---|
| 1991 | EPA completed a final assessment on the Toxicological Review for 2,2,4-Trimethylpentane. |
| 2006 | EPA began reviewing the Tox Assessment of TMP. While no new critical studies via the inhalation route were identified, newer mechanistic studies, gavage studies, and toxicokinetic studies were found. This information was used to update the assessment. |
| Dec 2006 | EPA released the external review draft for public review and comment. |
| Feb 2007 | EPA hosted an independent peer review panel workshop to discuss the draft report. |
| Jul 2007 | EPA released the final Toxicological Review of 2,2,4-Trimethylpentane : in support of the Integrated Risk Information System (IRIS). |
Status
Following the External Peer Review, the final assessment will be posted in 2007 in the IRIS database.Additional Information
Comments on the assessment may be submitted and reviewed using the e-Government Regulations.gov Web site. From the site, select Environmental Protection Agency and the keyword EPA-HQ-ORD-2006-1009 (for the docket ID).Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- Toxicological Review of 2,2,4-Trimethylpentane (PDF) (40 pp, 1.7 MB, about PDF)
- Charge to External Reviewers (PDF) (2 pp, 45.9 KB, about PDF)
- CONSOLIDATED COMMENTS FROM THE EXTERNAL PEER REVIEW (FINAL) (PDF) (11 pp, 75.2 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Federal Register Notices
Chemical Structure for
2,2,4-Trimethylpentane
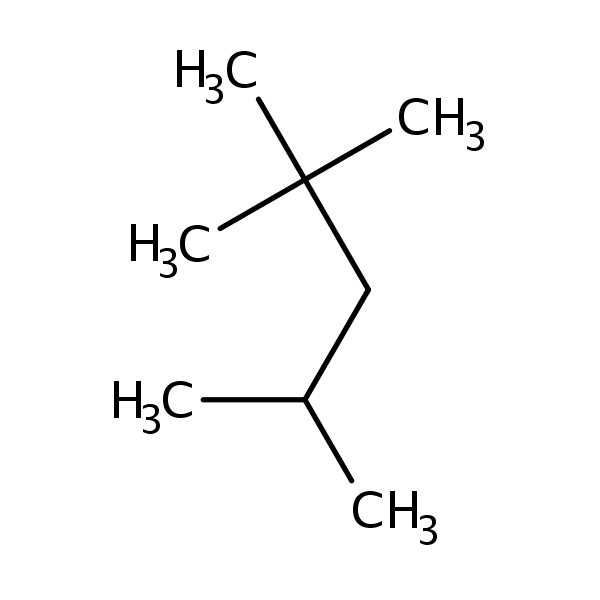
Synonyms
- AI3-23976
- HSDB 5682
- Iso-octane
- Isobutyltrimethylmethane
- Isooctane
- TMP
- Trimethylpentane
- pentane, 2,2,4-Trimethyl-
- 2,2,4-Trimethylpentane
- 2,4,4-Trimethylpentane
- 540-84-1
