Nitrobenzene
CASRN 98-95-3 | DTXSID3020964
- Toxicological Review (PDF) (250 pp, 3.7 MB, about PDF)
- IRIS Summary (PDF) (30 pp, 251.0 KB, about PDF)
IRIS Toxicological Review of Nitrobenzene (External Review Draft)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
EPA has conducted a peer review of the scientific basis supporting the human health hazard and dose-response assessments of nitrobenzene, that will appear on the Integrated Risk Information System (IRIS) database. Peer review is meant to ensure that science is used credibly and appropriately in derivation of the dose-response assessments and toxicological characterization.Nitrobenzene is used extensively in chemical manufacturing, especially in the synthesis of other industrial chemicals and intermediates. Most important among these is aniline. A re-assessment of the potential health effects of nitrobenzene has been undertaken. The draft assessment will present reference values for the noncancer effects (RfD and RfC) and a cancer assessment (IUR).
Background
IRIS is a database that contains information regarding potential adverse human health effects that may result from chronic (or lifetime) exposure to specific chemical substances found in the environment. The database (available in the Internet at http://ww/epa.gov/iris) contains qualitative and quantitative helath effects information for more than 540 chemical substances that may be used to support the first two steps of a risk assessment process. When supported by available data, the database provides oral reference doses (RfDs) and inhalation reference concentrations (RfCs) for chronic health effects, and oral slope factors and inhalation unit risks for carcinogenic effects.Combined with specific exposure information, government and private entities can use IRIS to help characterize public health risks of chemical substances in a site-specific situation and thereby support risk management decision designed to protect public health.
| Date | Description |
|---|---|
| Mar 2007 | EPA released the external review draft for public review and comment. |
| May 2007 | EPA hosted an independent peer review panel workshop to discuss the draft report. |
| Feb 2009 | EPA released the final Toxicological Review of Nitrobenzene: in support of the Integrated Risk Information System (IRIS). |
Status
Following the External Peer Review, the final assessment will be published on the IRIS database.Additional Information
Included for public review are the draft Toxicological Review for Nitrobenzene, a draft Appendix to the Toxicological Review, and a charge to external peer reviewers for EPA's health assessment of nitrobenzene. Comments on the assessment may be submitted and reviewed using the e-Government Regulations.gov Web site. From the site, select Environmental Protection Agency and the keyword EPA-HQ-ORD-2007-0241 (for the docket ID) to comment on this report.Download(s)
- Toxicological Review of Nitrobenzene (External Review Draft) (PDF) (169 pp, 2.0 MB, about PDF)
- APPENDIX B -1: DOSE-RESPONSE MODELING FOR DERIVATION OF AN RFD FOR NITROBENZENE (PDF) (62 pp, 407.2 KB, about PDF)
- Charge to External Peer Reviewers (PDF) (4 pp, 31.2 KB, about PDF)
- CONSOLIDATED COMMENTS FROM THE EXTERNAL PEER REVIEW (FINAL) (PDF) (43 pp, 222.7 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Federal Register Notices
Critical Effect Systems
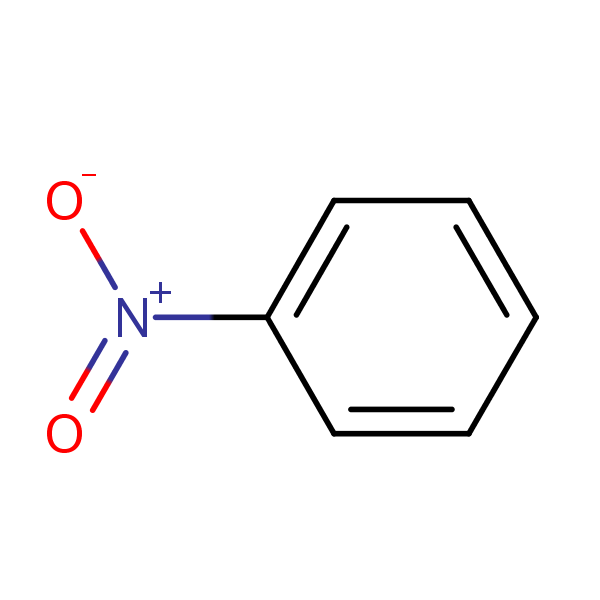
Chemical Structure for
Nitrobenzene
Synonyms
- Benzene, nitro-
- Essence of Mirbane
- Essence of Myrbane
- Mirbane oil
- NCI-c60082
- Nitrobenzene
- Nitrobenzol
- Oil of Mirbane
- Oil of Myrbane
- 98-95-3