Ethyl Tertiary Butyl Ether (ETBE)
CASRN 637-92-3 | DTXSID0025604
- Toxicological Review (PDF) (200 pp, 4.1 MB, about PDF)
- IRIS Executive Summary (PDF) (8 pp, 291.0 KB, about PDF)
- Supplemental Information on the IRIS Toxicological Review of Ethyl Tertiary Butyl Ether
IRIS Toxicological Review of Ethyl Tertiary Butyl Ether (ETBE) (External Review Draft, 2009)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
EPA is conducting a peer review and public comment of the scientific basis supporting the human health hazard and dose-response assessment of ethyl tertiary butyl ether (ETBE) that when finalized will appear on the Integrated Risk Information System (IRIS) database.Background
The draft Toxicological Review of Ethyl Tertiary Butyl Ether provides scientific support and rationale for the hazard and dose-response assessment pertaining to chronic exposure to ethyl tertiary butyl ether. Ethyl tertiary butyl ether is used as a fuel additive for gasoline to increase octane rating and has been used to meet air pollution reduction goals under the Clean Air Act. The use of ether oxygenates such as ethyl tertiary butyl ether in reformulated gasoline in the United States was effectively eliminated in 2006; however, use and production of these oxygenates has continued for the purpose of increasing octane rating in gasoline. Ethyl tertiary butyl ether has been used extensively in Europe and may be used as a substitute to replace the volume and oxygenate properties of methyl tertiary butyl ether, an oxygenate widely used in the United States prior to 2006. Plastic plumbing pipe, particularly silane cross-linked polyethylene (PEX), represents an additional potential source of ethyl tertiary butyl ether in drinking water.| Date | Description |
|---|---|
| 2004 | The IRIS Assessment of ethyl tertiary butyl ether was initiated in 2004. |
| Aug 2009 | EPA released the ETBE external review draft for public review and comment. |
| Oct 2009 | EPA announces a public listening session to be held on October 7, 2009 as explained in the September 23, 2009 Federal Register Notice. |
| Dec 2009 | EPA announces the details of an external peer review workshop to be held on January 26, 2010 in a December 22, 2009 Federal Register Notice. |
| Mar 2010 | EPA released the comments from the public comment period and external review workshop. Development of this draft was then halted pending the completion of several new studies that were identified during the peer review. |
Status
This draft has been archived.Additional Information
Note: On August 31, 2009, EPA posted an updated version of the report that includes an update on pages 40-45 of 9 missing lines due to a figure placement. Please contact the email or phone listed under contacts for more specific information.Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- IRIS Toxicological Review of Ethyl Tertiary Butyl Ether: In Support of the Summary Information in the Integrated Risk Information System (IRIS) (PDF) (335 pp, 1.7 MB, about PDF)
- Charge to Reviewers (PDF) (2 pp, 13.7 KB, about PDF)
- Consolidated Comments from the External Peer Review (Final) (PDF) (73 pp, 447.3 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
- 1. Principal Study: Centre International de Toxicologie (CIT, 2004b) Cover Letter (PDF) (1 pp, 55.7 KB, about PDF)
- 2. Principal Study: Centre International de Toxicologie (CIT, 2004b) Two-generation study (PDF) (1724 pp, 15.0 MB, about PDF)
- 3. Supporting Study: Centre International de Toxicologie (CIT, 2004a) Cover Letter (PDF) (1 pp, 375.0 KB, about PDF)
- 4. Supporting Study: Centre International de Toxicologie (CIT, 2004a) Prenatal developmental toxicity study (PDF) (280 pp, 5.0 MB, about PDF)
- 5. External Peer Review of Two 2004 Study Reports (Study No. 24860RSR and Study No. 24859-RSR) on Ethyl Tertiary Butyl Ether (ETBE) Performed by CIT (Centre International de Toxicologie) Under Contract for TOTAL France (PDF) (63 pp, 805.7 KB, about PDF)
- Update on Ramazzini Institute Date in IRIS Assessments
Federal Register Notices
Docket
Comments on the assessment may be submitted and reviewed using the Docket ID EPA-HQ-ORD-2009-0229Related Links
Critical Effect Systems
Tumor Sites
Chemical Structure for
Ethyl Tertiary Butyl Ether (ETBE)
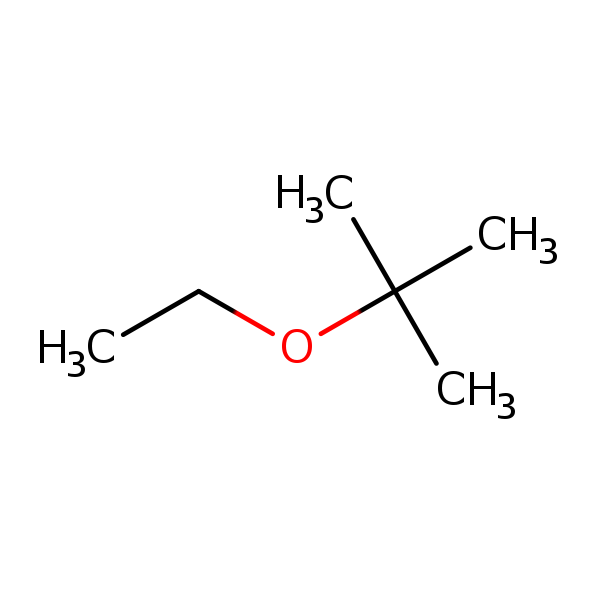
Synonyms
- ETBE
- ethyl 1,1-dimethylethyl ethyl ether
- ethyl tert-butyl ether
- ethyl tert-butyl oxide
- ethylene glycol t-butyl ether
- methyl-2-ethoxypropane
- tert-butyl ethyl ether
- 2-ethoxy-2-methylpropane
- 2-methyl-2-ethoxypropane
- 637-92-3