Ammonia
CASRN 7664-41-7 | DTXSID0023872
IRIS Toxicological Review of Ammonia Noncancer Inhalation (Interagency Science Discussion Draft)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
In September 2016, EPA finalized the IRIS assessment of Ammonia (Noncancer Inhalation). The Toxicological Review was reviewed internally by EPA and by other federal agencies and White House Offices before public release in June 2016. Consistent with the May 2009 IRIS assessment development process, all written comments on IRIS assessments submitted by other federal agencies and White House Offices are made publicly available. Accordingly, interagency comments and the interagency science discussion materials provided to other agencies, including interagency review drafts of the IRIS Toxicological Review of Ammonia (Noncancer Inhalation) are posted on this site.Note: No major science comments were received on the Interagency Science Discussion Draft.
Background
Ammonia occurs naturally in air, soil, and water. Ammonia is also produced by humans and other animals as part of normal biological processes. Ammonia is used as an agricultural fertilizer and in many cleaning products. Exposure to ammonia occurs primarily through breathing air containing ammonia gas, and may also occur via diet, drinking water, or direct skin contact. Concentrations of ammonia measured in ambient outdoor air range from 0.28‒15 μg/m3 and in indoor air from 0.09–166 μg/m3.Health effects of inhaled ammonia observed at levels exceeding naturally-occurring concentrations are generally limited to the respiratory tract, the site of direct contact with ammonia. Short-term inhalation exposure to high levels of ammonia in humans can cause irritation and serious burns in the mouth, lungs, and eyes. Chronic exposure to airborne ammonia can increase the risk of respiratory irritation, cough, wheezing, tightness in the chest, and reduction in the normal function of the lung in humans. Studies in experimental animals similarly indicate that breathing ammonia at sufficiently high concentrations can result in effects on the respiratory system. Animal studies also suggest that exposure to high levels of ammonia in air may adversely affect other organs, such as the liver, kidney, and spleen.
| Date | Description |
|---|---|
| May 1991 | The RfC for ammonia was posted to the IRIS database. |
| Feb 2012 | Revised draft assessment was submitted for Agency review and interagency science consultation. |
| Jun 2012 | EPA released the external review draft of the ammonia assessment for public review and comment. Additionally, the interagency science consultation draft, comments from reviewers, and EPA's responses to selected major interagency comments were also released. [Federal Register Notice Jun 8, 2012] |
| Aug 2013 | EPA's Science Advisory Board (SAB) announced a request for nominations for experts to augment the SAB Chemical Assessment Advisory Committee (CAAC) for the review of the EPA's draft IRIS assessment for ammonia [Federal Register Notice Aug 28, 2013] (3pp., 210Kb, about PDF), and made available the revised external review draft assessment and draft charge to peer reviewers. |
| Jul 2014 | EPA's SAB CAAC convenes to discuss the external review draft of the IRIS assessment. |
| Aug 2015 | EPA's IRIS Program receives final external peer review report from the SAB. To address SAB recommendations related to the assessment of ingested ammonia and to allow completion of the assessment of inhaled ammonia, the scope of the assessment was limited to the noncancer effects of ammonia via inhalation exposure. An assessment of ammonia (oral) was added to the IRIS Multi-Year Agenda in 2015. |
| Jun 2016 | EPA submits the revised draft for final Agency Review and Interagency Science Discussion. |
| Sep 2016 | EPA posts the final IRIS assessment of Ammonia—Noncancer Inhalation to the IRIS database. EPA also released the Interagency Science Discussion Draft, Comments and EPA's Response to Comments. |
Status
The ammonia (inhalation) assessment has been loaded into the IRIS Web site and database.Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- IRIS Toxicological Review of Ammonia -- Noncancer Inhalation (Interagency Science Discussion Draft) (PDF) (95 pp, 1.4 MB, about PDF)
- IRIS Toxicological Review of Ammonia -- Noncancer Inhalation (Interagency Science Discussion Draft) -- Supplemental Information (PDF) (131 pp, 1.7 MB, about PDF)
- 1. OMB Comments on Ammonia (PDF) (1 pp, 198.2 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
Related Links
Critical Effect Systems
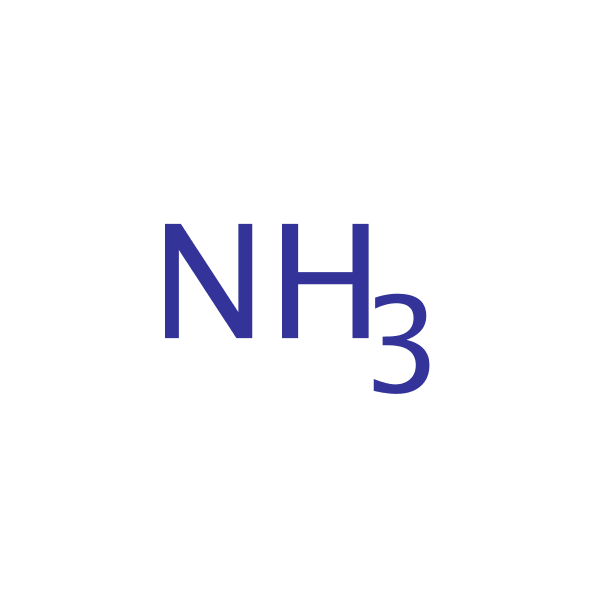
Chemical Structure for
Ammonia
Synonyms
- Am-Fol
- Ammonia
- Ammonia
- Ammonia gas
- Ammonia solution, strong
- Ammoniac [French]
- Ammoniaca [Italian]
- Ammoniak [German]
- Amoniaco [Spanish]
- Amoniak [Polish]
- Anhydrous ammonia
- Aromatic ammonia, vaporole
- Caswell No. 041
- EPA Pesticide Chemical Code 005302
- HSDB 162
- Nitro-sil
- R 717
- Spirit of Hartshorn
- UN 1005
- UN 2073
- UN 2672
- 7664-41-7