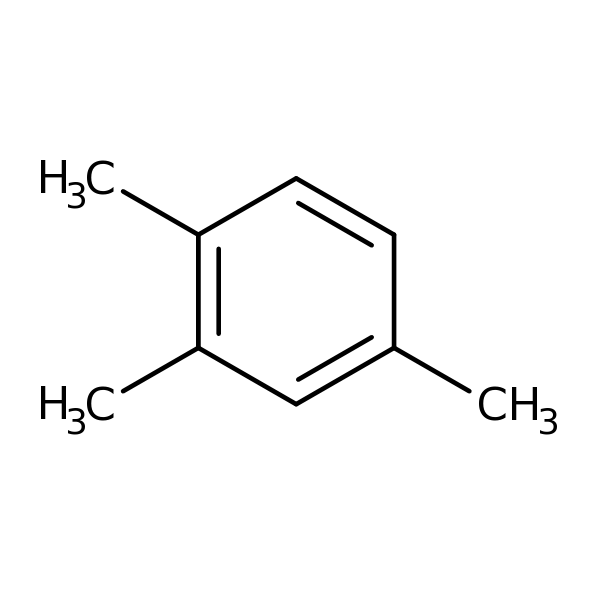
1,2,4-Trimethylbenzene
CASRN 95-63-6 | DTXSID6021402
- Toxicological Review (PDF) (150 pp, 2.5 MB, about PDF)
- IRIS Executive Summary (PDF) (10 pp, 315.4 KB, about PDF)
- Supplemental Information on the IRIS Toxicological Review of Trimethylbenzene
IRIS Toxicological Review of Trimethylbenzenes (Interagency Science Discussion Draft)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
In September 2016, EPA finalized the IRIS assessment of Trimethylbenzenes. The Toxicological Review was reviewed internally by EPA and by other federal agencies and White House Offices before public release in June 2016. Consistent with the May 2009 IRIS assessment development process, all written comments on IRIS assessments submitted by other federal agencies and White House Offices are made publicly available. Accordingly, interagency comments and the interagency science discussion materials provided to other agencies, including interagency review drafts of the IRIS Toxicological Review of Trimethylbenzenes are posted on this site.Background
Trimethylbenzenes (TMBs) are a commercially available mixture of three individual isomers: 1,2,3-TMB, 1,2,4-TMB, and 1,3,5-TMB. TMB isomers are produced during petroleum refining and production of aromatic hydrocarbons with nine carbons (i.e., C9 aromatic fraction). As the vast majority of the C9 fraction is used as a component of gasoline, vehicle emissions are expected to be the major anthropogenic source of TMBs. TMBs are volatile hydrocarbons, and humans are thus exposed to these isomers primarily through breathing air containing TMB vapors, although ingestion through food or drinking water is also possible.Effects on the nervous, respiratory, and hematological (i.e., blood) systems have been reported in occupationally- and residentially-exposed humans, but these effects were observed following exposure to complex mixtures containing TMB isomers, thus making it difficult to determine the contribution of each TMB isomer to the observed health effects. Health effects that are roughly analogous to those seen in humans have been observed in animals exposed to the individual isomers. Effects on the nervous system, including cognitive effects and decreased pain sensitivity, are the most widely observed effects in animals. Effects on other systems, including the respiratory and hematological systems, have also been observed in animals. Both 1,2,4-TMB and 1,3,5-TMB have been observed to elicit effects on pregnant animals and developing fetuses, but at exposure levels greater than those that cause effects on the nervous system. There is inadequate information to evaluate the carcinogenicity of TMBs.
| Date | Description |
|---|---|
| Jan 2012 | EPA submits the draft assessment for Interagency Science Consultation. In February, EPA hosted an interagency science consultation meeting on the review of the draft Toxicological Review of Trimethylbenzenes. |
| Jun 2012 | EPA released the external peer review draft of the trimethylbenzenes assessment for public review and comment. Additionally, the interagency science consultation draft, comments from reviewers, and EPA's responses to selected major interagency comments were also released (see "related links"). [Federal Register Notice June 29, 2012] |
| Aug 2013 | EPA's Science Advisory Board (SAB) announced a request for nominations for experts to augment the SAB Chemical Assessment Advisory Committee (CAAC) for the review of the EPA's draft IRIS assessment for trimethylbenzenes. [Federal Register Notice Aug 28, 2013](3pp., 210KB, about PDF), and made available the revised external review draft assessment and draft charge to peer reviewers. |
| Jan 2014 | EPA's SAB announced a 3-day public peer review meeting for the draft IRIS Toxicological Review of Trimethylbenzenes for Feb 18-20, 2014. [Federal Register Notice Jan 31, 2014] |
| Feb 2014 | To provide the public with additional time to prepare, EPA SAB staff office is rescheduling the February 18-20 meeting of the SAB Chemical Assessment Advisory Committee (CAAC). The EPA SAB staff office will announce a new date in the near future. |
| Mar 2014 | EPA's SAB Staff Office has announced two meetings for the SAB CAAC-Trimethylbenzenes panel for the review of the draft IRIS assessment of trimethylbenzenes. A public teleconference is scheduled for May 22, 2014. A face-to face panel meeting is scheduled for June 17-19, 2014 in Arlington, VA. [Federal Register Notice Mar 25, 2014] |
| Mar 2014 | EPA updated the peer review charge to include a question regarding EPA's assessment revisions and response to public comments. |
| May 2014 | EPA's SAB posted the agenda to the public teleconference meeting that will take place on May 22, 2014. See the SAB site for details. |
| Jun 2014 | EPA's SAB CAAC convenes to discuss the external review draft of the IRIS assessment. |
| Jun 2016 | EPA submits the revised interagency science discussion draft of the Toxicological Review of Trimethylbenzenes for final Agency review and Interagency science discussion. |
| Sep 2016 | EPA posts the final IRIS assessment of Trimethylbenzenes to the IRIS database. |
Status
Now that the interagency review draft has been completed and finalized, the TMB assessment has been loaded into the IRIS Web site and Database.Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- IRIS Toxicological Review of Trimethylbenzes (Interagency Science Discussion Draft) (PDF) (148 pp, 2.4 MB, about PDF)
- IRIS Toxicological Review of Trimethylbenzes (Interagency Science Discussion Draft) -- Supplemental Information (PDF) (353 pp, 9.6 MB, about PDF)
- EPA's Response to select Interagency Science Discussion Comments (PDF) (2 pp, 120.1 KB, about PDF)
- 1. DoD Comments on Trimethylbenzenes (PDF) (8 pp, 63.5 KB, about PDF)
- 2. OMB Email Comments on Trimethylbenzenes (PDF) (1 pp, 191.4 KB, about PDF)
- 3. OMB Comments on Trimethylbenzenes (PDF) (1 pp, 163.1 KB, about PDF)
- 4. SBA Comments on Trimethylbenzenes (PDF) (4 pp, 692.4 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
Related Links
Critical Effect Systems
Chemical Structure for
1,2,4-Trimethylbenzene
Synonyms
- asymmetrical trimethylbenzene
- mesitylene
- methylxylene
- pseudocumene
- psi-Cumene