Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX)
CASRN 121-82-4 | DTXSID9024142
IRIS Toxicological Review of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) (Final Report)
On this page:
Overview
EPA has finalized the Integrated Risk Information System (IRIS) assessment of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX). This assessment addresses the potential cancer and noncancer human health effects from exposure to RDX. Now final, this assessment updates the toxicological information on RDX posted to the IRIS database in 1988 and 1990. EPA’s program and regional offices may use this assessment to inform decisions to protect human health.Background
Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX, or Royal Demolition eXplosive) is a military explosive with limited civilian uses. In the United States, RDX is produced at Army munitions plants; it is not produced commercially.| Date | Description |
|---|---|
| Dec 2013 | EPA discussed preliminary draft materials at the December 12-13, 2013 IRIS Bimonthly Public Meeting. Presentations are also available on the meeting web site. |
| Mar 2016 | EPA released the public comment draft for a 60-day review period and announced it will be discussed at the May 10, 2016 IRIS Public Science Meeting. EPA also released the interagency science consultation documents, comments from reviewers, and EPA's response to major comments. [Federal Register Notice: Mar 10, 2016] |
| Mar 2016 | EPA announced a request for nominations of experts to augment the SAB CAAC for the review of the draft RDX assessment. Any related questions should be directed to the SAB. [SAB Federal Register Notice Mar 18, 2016] |
| May 2016 | EPA discussed the draft RDX Toxicological Review at the May IRIS Public Science Meeting. |
| Sep 2016 | EPA released the external review draft for peer review by EPA's Science Advisory Board (SAB) Chemical Assessment Advisory Committee (CAAC). Any related questions should be directed to the SAB. [SAB Federal Register Notice Sep 29, 2016] |
| Dec 2016 | EPA's Science Advisory Board (SAB) Chemical Assessment Advisory Committee (CAAC) convened a 3-day meeting to conduct a peer review of the draft Toxicological Review. |
| Apr 2017 | EPA's Science Advisory Board (SAB) held a 2-day public external peer review teleconference to discuss the draft Toxicological Review. [Federal Register Notice Mar 22, 2017] |
| May 2018 | EPA submitted a revised draft for final Agency Review and Interagency Science Discussion. |
| Aug 2018 | EPA posted the final IRIS Toxicological Review of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) and summary to the IRIS database. |
Download(s)
This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
- IRIS Toxicological Review of Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) (EPA/635/R-18/211Fa) (PDF) (187 pp, 2.4 MB, about PDF)
- IRIS Toxicological Review of Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) -- Supplemental Information (EPA/635/R-18/211Fb) (PDF) (216 pp, 2.6 MB, about PDF)
- IRIS Toxicological Review of Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) -- Executive Summary (EPA/635/R-18/211Fc) (PDF) (7 pp, 870.1 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
- IRIS Toxicological Review of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) (Preliminary Assessment Materials)
- IRIS Toxicological Review of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) (Public Comment Draft)
- IRIS Toxicological Review of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) (Interagency Science Consultation Draft)
- IRIS Toxicological Review of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) (External Review Draft)
- IRIS Toxicological Review of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) (Interagency Science Discussion Draft)
Related Links
Critical Effect Systems
Tumor Sites
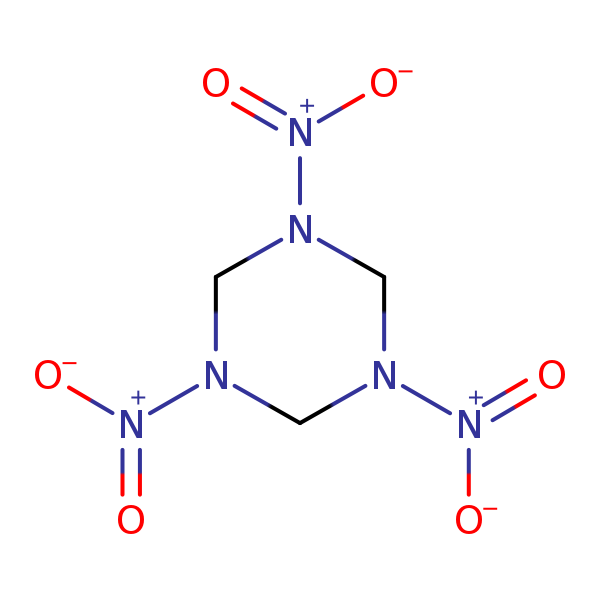
Chemical Structure for
Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX)
Synonyms
- Cyclonite
- Cyclotrimethylenenitramine
- Cyclotrimethylenetrinitramine
- Cyklonit
- Esaidro-1,3,5-trinitro-1,3,5-triazina
- Giekfol
- Heksoflen
- Heksogen
- Hexahydro-1,3,5-trinitro-1,3,5-triazin
- Hexahydro-1,3,5-trinitro-1,3,5-triazine
- Hexahydro-1,3,5-trinitro-s-triazine
- Hexogeen
- Hexogen
- Hexogen 5w
- Hexolite
- NSC 312447
- PBX(AF) 108
- PBX-MVF
- PBXN(AF) 108
- PBXW 108(e)
- RDX
- Research Development Explosive
- Royal Demolition eXplosive
- T4
- Trimethyleentrinitramine
- Trimethylenetrinitramine
- Trinitrocyclotrimethylene triamine
- UN 0072
- UN 0483
- perhydro-1,3,5-trinitro-1,3,5-triazine; RDX
- s-Triazine, hexahydro-1,3,5-trinitro-
- sym-Trimethylenetrinitramine
- trinitrotrimethylenetriamine
- 1,3,5-Triazine, hexahydro-1,3,5-trinitro-
- 1,3,5-Trinitro-1,3,5-triazacyclohexane
- 1,3,5-Trinitrohexahydro-s-triazine
- 1,3,5-triaza-1,3,5-trinitrocyclohexane
- 1,3,5-trinitro-1,3,5-triazinane
- 1,3,5-trinitrohexahydro-1,3,5-triazine
- 1,3,5-trinitroperhydro-1,3,5-triazine
- 121-82-4