Chloroprene
CASRN 126-99-8 | DTXSID5020316
- Toxicological Review (PDF) (303 pp, 4.4 MB, about PDF)
- IRIS Summary (PDF) (30 pp, 299.8 KB, about PDF)
- Systematic Review of Chloroprene (Jan 2018)
External Peer Review of a Report on Physiologically Based Pharmacokinetic (PBPK) Modeling for Chloroprene and a Supplemental Analysis of Metabolite Clearance (July 2020)
Alert
Notice
[NOTICE] Versar, an EPA contractor, has completed the external peer review of a Report on Physiologically Based Pharmacokinetic (PBPK) Modeling for Chloroprene and a Supplemental Analysis of Metabolite Clearance (July 2020). EPA has posted the final peer review report and meeting presentations under “Meeting Materials.”
On this page:
Meeting Agenda
Meeting Objective
On July 24, EPA announced an independent peer review on the draft documents, Physiologically Based Pharmacokinetic (PBPK) Modeling for Chloroprene (Ramboll, 2020) and Supplement: Uncertainty Analysis of In Vitro Metabolic Parameters and of In Vivo Extrapolation (IVIVE) Used in a Physiologically Based Pharmacokinetic (PBPK) Model for Chloroprene (U.S. EPA, 2020). It will be conducted by Versar, an EPA contractor for the external scientific peer review. EPA will also convene a public meeting as a part of this peer review to allow the public an opportunity to provide oral comments to the peer reviewers. Written comments will be solicited and will be provided to the external peer reviewers. Written comments can be provided in the docket (Id Number EPA-HQ-ORD-2020-0181).
Dates
Virtual Public Meeting: Will be held on October 5 and 6, 2020, from 9:00 a.m. to 5:00 p.m. You must register online to receive the webcast meeting link and audio teleconference information for participation. [https://www.eventbrite.com/e/epa-public-meeting-external-peer-review-for-pbpk-modeling-for-chloroprene-registration-109878740270]
- Meeting Agenda - Chloroprene PBPK Virtual Meeting Final Agenda (PDF) (2pp, 187Kb)
- Meeting Date - October 5 and October 6, 2020
- Deadline - General Registration: October 1, 2020
- Deadline - Register to Provide Oral Comment: October 1, 2020
Registration Instructions
To attend the peer review virtual meeting register no later than October 1, 2020. There will be a limited amount of time for oral statements from the public near the beginning of the peer review meeting on the first day. If you wish to make an oral statement during the meeting, you must notify Versar of your request to speak no later than October 1, 2020. Versar will notify speakers of specific time limits for their oral statements. Versar will accept requests to make oral statements on a first-come, first-served basis, and may limit the amount of time for each speaker as well as the number of speakers due to time constraints.
Background
On June 26, 2017, the U.S. EPA received a Request for Correction (RFC) provided on behalf of Denka Performance Elastomer LLC (DPE). In the RFC letter, DPE states that the Toxicological Review of Chloroprene (CAS NO. 126-99-8) in Support of Summary Information on the Integrated Risk Information System (IRIS), disseminated by EPA’s Office of Research and Development (ORD) in 2010, does not reflect the “best available science” or “sound and objective scientific practices” and requested correction. On January 25, 2018, EPA concluded in response that the underlying information and conclusions presented in the IRIS Chloroprene assessment are consistent with the EPA’s Information Quality Guidelines and that no evidence published since the 2010 IRIS Assessment would change its conclusions. On July 23, 2018, DPE submitted a Request for Reconsideration of Denial of Request for Correction (RFR) with regards to EPA’s decision and entered discussions with EPA to address the uncertainties identified by EPA as documented in the RFC response.
More information regarding the Chloroprene RFC/RFR can be found on the EPA's Information Quality Guideline website. Discussions with Denka LLC and its contractor, Ramboll, are summarized on the IRIS website.
An updated PBPK model is now available in a report: “Physiologically Based Pharmacokinetic (PBPK) Modeling for Chloroprene (Ramboll, 2020)." This report and the Supplemental Analysis of Metabolite Clearance (U.S. EPA, 2020) document will undergo external peer review to help to inform future decisions regarding the RFR.
Peer Review Event Point of Contact (POC):
- For information regarding the peer review contact: ChloroprenePBPK@versar.com. Please include the subject line "Chloroprene PBPK Peer Review" with your comment or contact by phone at (301) 304-3121 and ask for Ms. Tracey Cowen.
Meeting Materials
- Final Chloroprene Post-Meeting Peer Review Summary Report (PDF) (192 pp, 2.7 MB, about PDF)
- Background Description for Chloroprene PBPK Modeling July 2020 (U.S. EPA, 2020) (PDF) (9 pp, 267.6 KB, about PDF)
- U.S. EPA Supplement: Uncertainty Analysis of In Vitro Metabolic Parameters and of In Vitro to In Vivo Extrapolation Used in a Physiologically Based Pharmacokinetic Model for Chloroprene (EPA, 2020) (PDF) (24 pp, 490.2 KB, about PDF)
- Chloroprene PBPK Virtual Meeting Final Agenda (PDF) (2 pp, 182.6 KB, about PDF)
- 1. EPA Introduction to the Chloroprene Meeting (PDF) (4 pp, 246.1 KB, about PDF)
- 2. Supplemental Analysis of Parameter Uncertainty (Overview) (PDF) (13 pp, 695.3 KB, about PDF)
- 3. Ramboll PBPK Model and Model Validation (Presentation) (PDF) (30 pp, 1.0 MB, about PDF)
- Charge Questions for Reviewers (U.S. EPA, 2020) (PDF) (7 pp, 256.8 KB, about PDF)
- List of Final Peer Reviewers for Chloroprene PBPK Peer Review Aug 2020 (PDF) (2 pp, 19.5 KB, about PDF)
- List of Candidates for Chloroprene PBPK Peer Review July 2020 (PDF) (3 pp, 65.4 KB, about PDF)
- Report on Physiologically Based Pharmacokinetic (PBPK) Modeling for Chloroprene (Ramboll, 2020) (PDF) (35 pp, 749.0 KB, about PDF)
- Supp Mat A: Supplemental Tables (Ramboll, 2020) (PDF) (9 pp, 215.8 KB, about PDF)
- Supp Mat B: Re-estimation of Metabolism Parameters (Ramboll, 2020) (PDF) (17 pp, 947.3 KB, about PDF)
- Supp Mat C: IVIVE Literature Review (Ramboll, 2020) (PDF) (10 pp, 408.2 KB, about PDF)
- Supp Mat D: Metabolism Parameter Calculations (Ramboll, 2020)
- Supp Mat E: PBPK Model Equations (Ramboll, 2020) (PDF) (7 pp, 134.8 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Meeting Related Link(s)
- Systematic Review of Chloroprene [CASRN 126-99-8] Studies Published Since 2010 IRIS Assessment to Support Consideration of the Denka Request for Correction (RfC)
- IRIS Toxicological Review of Chloroprene (Final Report)
- EPA Information Quality Guidelines, Requests for Correction and Requests for Reconsideration, #17002
HERO Project
Federal Register Notices
Docket
Comments on the assessment may be submitted and reviewed using the Docket ID EPA-HQ-ORD-2020-0181Critical Effect Systems
Tumor Sites
Chemical Structure for
Chloroprene
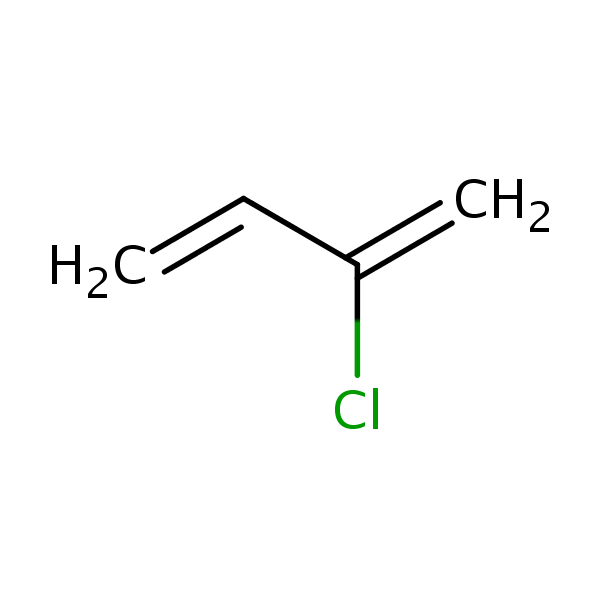
Synonyms
- beta-chloroprene
- chlorobutadiene
- chloroprene
- 126-99-8
- 2-chloro-1,3-butadiene
- 2-chlorobuta-1,3-diene
- 2-chlorobutadiene
- 2-chlorobutadiene-1,3