Formaldehyde
CASRN 50-00-0 | DTXSID7020637
- Toxicological Review (PDF) (927 pp, 16.3 MB, about PDF)
- IRIS Executive Summary (PDF) (10 pp, 450.2 KB, about PDF)
IRIS Program Outlook April 2019-Suspended/Discontinued Assessment
Overview
In April 2019, the IRIS Program announced under the Program Outlook that in an effort to modernize its workflow, IRIS has moved away from one-size-fits-all assessments to a portfolio of chemical evaluation products to meet specific decision needs. This approach optimizes the application of best practices of systematic review in the IRIS Program.As a result, EPA prioritized its IRIS assessments, originally announced in 2018, to meet the highest needs of EPA Programs and Regions and to bring greater focus to assessments actively under development (see December 2018 IRIS Program Outlook) and IRIS assessments for hexabromocyclododecane (HBCD), acrylonitrile, n-butyl alcohol, and phthalates (butyl benzyl phthalate, dibutyl phthalate, diethyl phthalate, di-isobutyl phthalate, and di-isononyl phthalate) were discontinued. New or updated assessments for these would not be added to the IRIS database at this time. Other assessments that were not identified as priorities for fiscal year 2019 were suspended but may be restarted as Agency priorities change. These include ammonia, chloroform, ethylbenzene, formaldehyde, manganese, naphthalene, nitrite/nitrate, PAH mixtures, and uranium. Draft assessment materials previously released on the IRIS website will remain accessible for reference on individual chemical pages. Additionally, existing toxicity values found on IRIS will remain available for use. More information about these chemicals can be found on the IRIS A to Z list.
Download(s)
- IRIS Program Outlook (Apr 2019) (PDF) (3 pp, 124.0 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Related Links
Critical Effect Systems
Tumor Sites
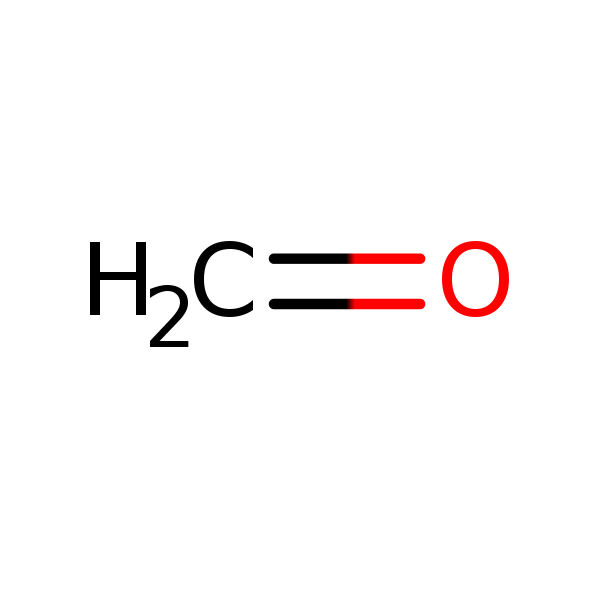
Chemical Structure for
Formaldehyde
Synonyms
- Aldehyd mravenci (Czech)
- Aldehyde formique (French)
- Aldeide formica (Italian)
- BFV
- FA
- Formaldehyd (Czech, Polish)
- Formaldehyde
- Formaldehyde solution (DOT)
- Formalin
- Formalith
- Formic aldehyde
- Formol
- Fyde
- Hoch
- Ivalon
- Karsan
- Lysoform
- Methanal
- Methyl aldehyde
- Methylene glycol
- Methylene oxide
- Morbicid
- NCI-C02799
- Oplossingen (Dutch)
- Oxomethane
- Oxymethylene
- Paraform
- Polyoxymethylene glycols
- RCRA Waste Number U122
- Superlysoform
- UN 1198 (DOT)
- UN 2209 (DOT)
- 50-00-0