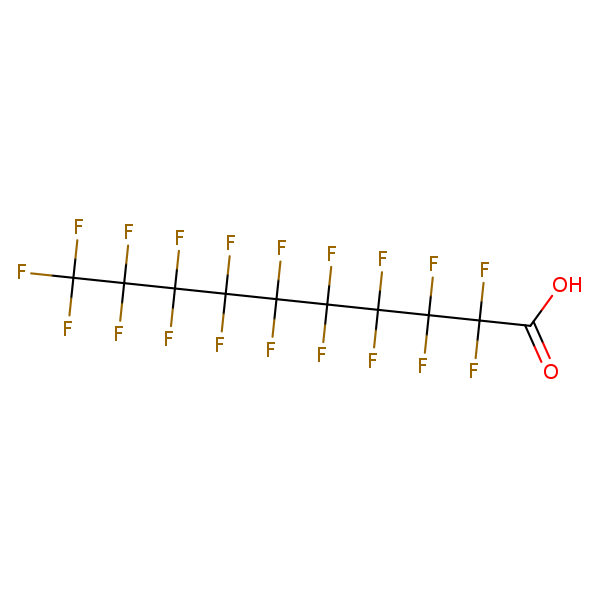
Perfluorodecanoic Acid (PFDA)
CASRN 335-76-2 | DTXSID3031860
- IRIS Executive Summary (PDF) (7 pp, 393.6 KB, about PDF)
External Peer Review Activities for PFDA Integrated Risk Information System (IRIS) Assessment (Jul 2023)
On this page:
Meeting Details
Per- and polyfluoroalkyl substances (PFAS) are a large class of synthetic (man-made) chemicals widely used in consumer products and industrial processes. The basic structure of PFAS consists of a carbon chain surrounded by fluorine atoms, with different chemicals possessing different end groups; thousands of distinct PFAS exist in commerce.
Meeting Agenda
Meeting Objective
To facilitate the independent external peer review of the draft PFAS IRIS assessments, U.S. Environmental Protection Agency (EPA) contracted with ERG to run a public meeting. This website serves as a resource for the peer review activities associated with the draft IRIS assessment of PFDA.
To help address this complex issue, the EPA is taking a proactive approach. Specifically, the development of human health toxicity assessments for exposure to individual PFAS represents only one component of the broader PFAS action plan underway at the EPA. The five Integrated Risk Information System (IRIS) toxicity assessments are being developed according to the scope and methods outlined in the systematic review protocol building upon several other PFAS assessments that have already been developed.
Meeting Details
Dates
- Monday, July 10, 2023: 10:00 AM - 4:00 PM EDT
- Tuesday, July 11, 2023: 10:00 AM - 4:00 PM EDT
- Thursday, July 13, 2023: 11:30 AM - 2:15 PM EDT
Registration
The peer review meeting will take place via Zoom and will be open to anyone to attend as an observer. The meeting will include a brief public comment period.
- Webinar Registration - Zoom (zoomgov.com)
- EPA External Peer Review Meeting (erg.com)
- Contact for questions regarding the peer review: laurie.waite@erg.com (subject line: PFDA Peer Review Meeting).
Panel Details
Member Details
- John L. Adgate, Ph.D., MSPH; Professor, University of Colorado
- Courtney C. Carignan, Ph.D.; Professor, Michigan State University
- Elaine M. Faustman, Ph.D., DABT; Professor, University of Washington
- Jeffrey W. Fisher, Ph.D.; Senior Research Fellow, ScitoVation
- Panagiotis G. Georgopoulos, Ph.D.; Professor, Rutgers University
- Joseph T. Haney, M.S.; Distinguished Toxicologist, Texas Commission on Environmental Quality (TCEQ)
- Alan M. Hoberman, Ph.D., DABT; President, Argus International, Inc.
- Angela M. Leung, MD.; Clinical Associate Professor of Medicine, UCLA
- R. Thomas Zoeller, Ph.D.; Professor Emeritus, University of Massachusetts Amherst
Formation Process
- Federal Register Notice: Request for Nominations of Experts for the Review of EPA's Draft Toxicological Reviews of Perfluorodecanoic Acid (PFDA), Perfluorononanoic Acid (PFNA), Perfluorohexanoic Acid (PFHxA), Perfluorohexanesulfonic acid (PFHxS), and Perfluorobutanoic acid (PFBA)
- List of Candidates for IRIS PFAS Peer Review
Additional Information
For more information regarding registration or the peer review meeting, please contact the ERG, Inc. at peerreview@erg.com (subject line: EPA PFAS assessments peer review). ). If you have a disability that interferes with your ability to access the information on this page or with participation in the Peer Review Meeting, please contact laurie.waite@erg.com (subject line: PFDA Peer Review Meeting) and let us know the nature of the accessibility problem and the preferred format that you want to receive the information (electronic format (e.g., ASCII, standard print, large print, etc.).
Meeting Materials
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- Final Meeting Agenda for the PFDA External Peer Review Meeting (July 2023) (PDF) (2 pp, 40.0 KB, about PDF)
- External Panel Peer Review of EPA's Draft "IRIS Toxicological Review of Perfluorodecanoic Acid (PFDA) and Related Salts" Final Peer Review Report (Oct 2023) (PDF) (200 pp, 3.1 MB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Registration
Related Links
Critical Effect Systems
Chemical Structure for
Perfluorodecanoic Acid (PFDA)