Naphthalene
CASRN 91-20-3 | DTXSID8020913
- Toxicological Review (PDF) (116 pp, 551.8 KB, about PDF)
- IRIS Summary (PDF) (32 pp, 99.6 KB, about PDF)
- Status: naphthalene is in step 1 at this time.
Summary Review of Health Effects Associated with Naphthalene: Health Issue Assessment (1987)
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
Naphthalene is released into ambient air via industrial gaseous and particulate emissions, tobacco use, and through consumer use. The data base concerning exposure of humans via inhalation and associated health effects is virtually nonexistent. Overexposure often results in acute hemolytic anemia and has been associated with cataract formation. There are no available dose-response data. In laboratory animals, two principal target tissues have been identified: non-ciliated bronchiolar epithelial (Clara) cells and eye tissue. The metabolite(s) that is responsible for Clara cell damage is unknown. There are no published studies involving inhalation exposure.Administration of naphthalene by routes other than inhalation has been shown to produce cataracts in rats, rabbits, and one mouse strain. Animal strains with pigmented eyes develop cataracts faster and more severely than albino strains. The likely causative agent is polyphenol oxydase, found only in pigmented eyes, that catalyzes the formation of 1,2-naphthoquione which binds to lens tissue. Negative results have been reported for gene mutations (Salmonella), unscheduled DNA synthesis in rat hepatocytes and microneuclei in mouse bone marrow. Limited teratology studies in rats and rabbits reported no gross abnormalities. In a single dose (300 mg/kg) study in mice, both maternal and fetal toxicity were reported.
Download(s)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Quick Check

Critical Effect Systems
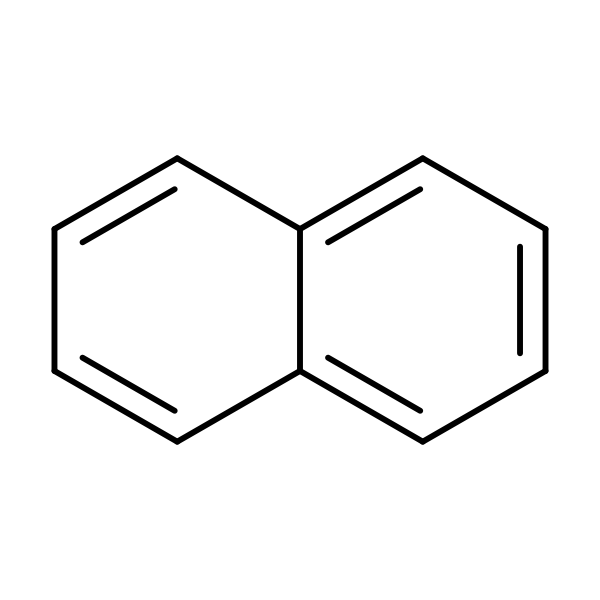
Chemical Structure for
Naphthalene
Synonyms
- Albocarbon
- Dezodorator
- EPA Pesticide Chemical Code 055801
- HSDB 184
- Moth Balls
- Moth Flakes
- NCI-C52904
- NSC 37565
- Naftalen [Polish]
- Naftaleno [Spanish]
- Naphtalene [French]
- Naphthalene
- Naphthalene
- Naphthalin
- Naphthaline
- Naphthene
- Napthalene, molten
- RCRA Waste Number U165
- Tar Camphor
- UN 1334
- UN 2304
- White Tar
- caswell No. 587
- 91-20-3