1,1,1-Trichloroethane
CASRN 71-55-6 | DTXSID0021381
- Toxicological Review (PDF) (237 pp, 1.0 MB, about PDF)
- IRIS Summary (PDF) (55 pp, 377.0 KB, about PDF)
IRIS Toxicological Review and Summary Documents for 1,1,1-Trichloroethane (Peer Review Plan)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
EPA's assessment of the carcinogenic potential of 1,1,1-trichloroethane was entered into the IRIS database in 1988, and the assessment of noncancer effects following oral exposure was withdrawn from IRIS in 1991. The IRIS program prepared an update of the IRIS assessment for 1,1,1-trichloroethane, which incorporated health effects information published since the last assessment was prepared as well as new risk assessment methods.The IRIS assessment for 1,1,1-trichloroethane consists of a Toxicological Review and IRIS Summary. The Toxicological Review is a critical review of the physicochemical and toxicokinetic properties of the chemical and its toxicity in humans and experimental systems. The assessment presents reference values for the noncancer effects of 1,1,1-trichloroethane (Reference Dose and Reference Concentration), and a cancer assessment. The Toxicological Review and IRIS Summary were subject to internal peer consultation, Agency review, Interagency review, and external scientific peer review. The final products constitute the Agency's opinion on the toxicity of 1,1,1-trichloroethane. The assessment of 1,1,1-trichloroethane also served as a pilot for the implementation of certain health assessment procedures proposed in the 2002 report, "A Review of the Reference Dose and Reference Concentration Processes," prepared by the Risk Assessment Forum's Reference Dose/Reference Concentration (RfD/RfC) Technical Panel. Included in this pilot was the implementation of recommendations related to the development of toxicity values for less-than-lifetime exposure, the presentation of study findings in tabular and graphical formats, and the use of a weight-of-evidence approach for characterizing the potential for noncancer effects in exposed populations.
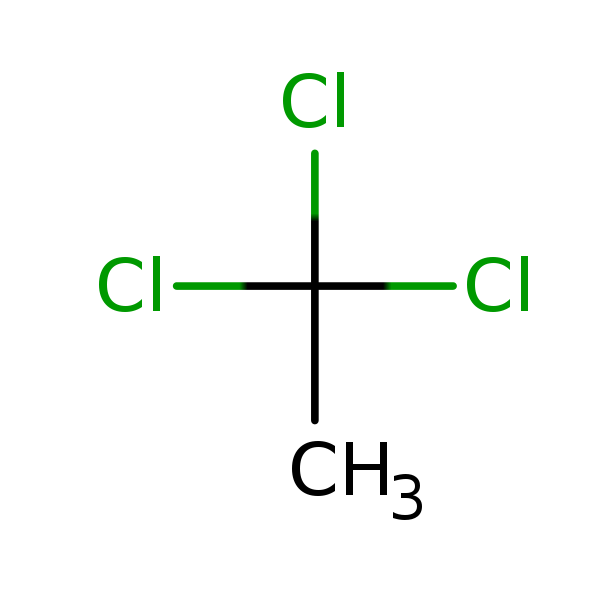
Chemical Structure for
1,1,1-Trichloroethane
Synonyms
- Aerothene MM
- Aerothene tt
- Algylen
- Alpha-t
- Baltana
- CF 2
- Chloroetene
- Chloroethane-NU
- Chloroethene
- Chloroethene nu
- Chloroform, methyl-
- Chlorothane nu
- Chlorothene
- Chlorothene SM
- Chlorothene nu
- Chlorothene vg
- Chlorten
- Chlorylen
- Dowclene LS
- Ethane, 1,1,1-trichloro-
- Gemalgene
- Genklene LB
- ICI-CF 2
- Inhibisol
- Methylchloroform
- Methyltrichloromethane
- NCI-C04626
- RCRA Waste Number U226
- Solvent 111
- TCEA
- Trichloran
- Trichloroethane, 1,1,1-
- Trichloromethylmethane
- Trielene
- UN 2831
- alpha-Trichloroethane
- tri-Ethane
- 1,1,1-TCA
- 1,1,1-TCE
- 71-55-6


