Methylmercury (MeHg)
CASRN 22967-92-6 | DTXSID9024198
- IRIS Summary (PDF) (44 pp, 252.0 KB, about PDF)
- Status: methylmercury (mehg) is in step 1 at this time.
IRIS Summary Update (2001) and EPA Guidelines for Methylmercury (2009 & 2010)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
In January 2001, U.S. EPA finalized the guidance for methylmercury in the Water Quality Criterion for the Protection of Human Health: Methylmercury, Final for states and authorized tribes.
Then eight years later, EPA released new guidance under the Methylmercury Implementation Guidance, but the following year the previous Obama Administration asked EPA to confirm the continued appropriateness and applicability of several agency actions, including this guidance. Therefore, based on the outcome of this review, in April 2010, EPA improved the document's clarity, and as a result, the ability of states and tribes to protect public health from methylmercury impacts by issuing a 2010 update, but this did not change the fundamental policy recommendations from the original January 2009 document.
Background
Methylmercury is a highly toxic substance; there are a number of adverse health effects associated with methylmercury exposure. Most extensive are the data for neurotoxicity, particularly in developing organisms. Therefore the brain is considered to be the most sensitive target organ for which there are data suitable for derivation of an RfD.The National Research Council (NRC) considered three epidemiological longitudinal developmental studies suitable for quantitative risk assessment: the Seychelles Islands, the Faroe Islands, and New Zealand. The Seychelles study has yielded no evidence of impairment related to methylmercury exposure, while both the other studies have found dose-related adverse effects on a number of neuropsychological endpoints. The Faroe Islands study, the larger of the latter two studies, has been extensively peer-reviewed and was used for the derivation of the RfD. The NRC's major finding was that the results of the Faroe Islands study provide scientifically credible basis on which to base EPA's RfD value.
Download(s)
This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
- IRIS Summary for Methylmercury (PDF) (43 pp, 253.9 KB, about PDF)
- Methylmercury Implementation Guidance (Updated Apr 2010)
- Methylmercury Implementation Guidance (January 2009)
- Water Quality Criterion for the Protection of Human Health: Methylmercury, Final 2001 (PDF) (308 pp, 743.9 MB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Quick Check

Critical Effect Systems
Chemical Structure for
Methylmercury (MeHg)
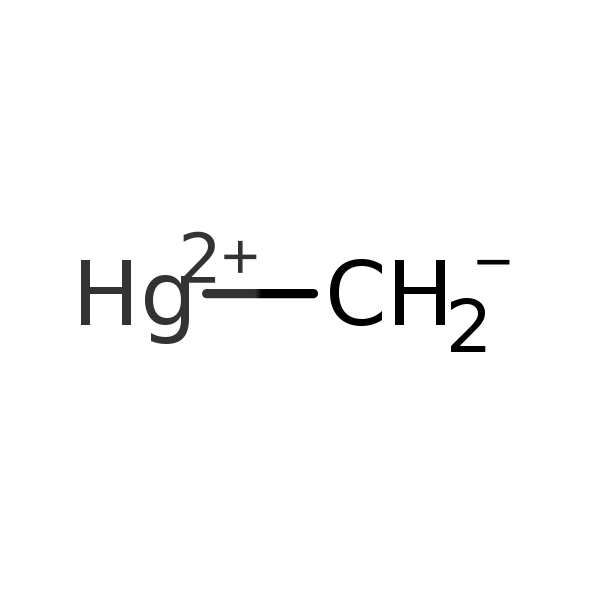
Synonyms
- MEHG
- Mercury (1+), methyl-, ion
- Mercury(1+), methyl-
- Methyl mercury
- Methylmercury
- Methylmercury (MeHg)
- Methylmercury (ii) cation
- Methylmercury ii
- Methylmercury ion
- 22967-92-6


