Polychlorinated Biphenyls (PCBs)
CASRN 1336-36-3 | DTXSID5024267
- IRIS Summary (PDF) (22 pp, 150 K)
- Status: Polychlorinated Biphenyls (PCBs) is in step 1 at this time; see Quick Check.
On this page:
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (22 pp, 150 K) Last Updated: 06/01/1994
Information reviewed but value not estimated.
Note: See the assessments under Related Links for information on the effects of oral exposure to specific PCB mixtures, including RfDs for Aroclor 1016 and Aroclor 1254.
Reference Concentration for Inhalation Exposure (RfC) (PDF) (22 pp, 150 K)
Not assessed under the IRIS Program.
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(22 pp, 150 K)
Last Updated: 10/01/1996
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| B2 (Probable human carcinogen - based on sufficient evidence of carcinogenicity in animals) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 1986) |
- A 1996 study found liver tumors in female rats exposed to Aroclors 1260, 1254, 1242, and 1016, and in male rats exposed to 1260. These mixtures contain overlapping groups of congeners that, together, span the range of congeners most often found in environmental mixtures. Earlier studies found high, statistically significant incidences of liver tumors in rats ingesting Aroclor 1260 or Clophen A 60 (Kimbrough et al., 1975 Norback and Weltman, 1985 Schaeffer et al., 1984). Mechanistic studies are beginning to identify several congeners that have dioxin-like activity and may promote tumors by different modes of action. PCBs are absorbed through ingestion, inhalation, and dermal exposure, after which they are transported similarly through the circulation. This provides a reasonable basis for expecting similar internal effects from different routes of environmental exposure. Information on relative absorption rates suggests that differences in toxicity across exposure routes are small. The human studies are being updated currently available evidence is inadequate, but suggestive.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (22 pp, 150 K)
Oral Slope Factor:
2
per mg/kg-day
(high risk and persistence, upper bound)
Extrapolation Method: Linear extrapolation below LED10s
Tumor site(s): Hepatic
Tumor type(s): Liver hepatocellular adenomas, carcinomas, cholangiomas, or cholangiocarcinomas (Brunner et al., 1996 Norback and Weltman, 1985)
Note: Cancer potency of PCB mixtures is determined by a tiered approach that depends on exposure matrix and route, type of congener present, and lifestage. See IRIS Summary for specific factors used to select appropriate slope factor. High risk and persistence: 2 per mg/kg-day; Low risk and persistence: 0.4 per mg/kg-day; Lowest risk and persistence: 0.07 per mg/kg-day.
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (22 pp, 150 K)
Inhalation Unit Risk:
1
x 10-4
per µg/m3
(Applies to inhalation of evaporated congeners)
Extrapolation Method: Linear extrapolation below LED10; route-to-route extrapolation from the oral slope factor
Tumor site(s): Hepatic
Tumor type(s): Liver hepatocellular adenomas, carcinomas, cholangiomas, or cholangiocarcinomas (Brunner et al., 1996; Norback and Weltman, 1985)
Note: For inhalation of an aerosol or dust contaminated with PCBs, see the IRIS Summary for information on the appropriate inhalation unit risk.
Program Outlook Details
| Public Assessment Materials | Date |
|---|---|
| Problem Formulation Materials/IRIS Assessment Plan | Apr-2015 |
| Preliminary Assessment Materials/Systematic Review Protocol | Dec-2019 |
| Public Comment | TBD |
| External Peer Review | TBD |
| Post Final Assessment | TBD |
Note: Any future dates displayed in the table above should be considered estimates and are subject to change. Once the external peer review is complete, estimated dates for release of the final assessment will be published. For the latest information on the status of this assessment, please refer to the IRIS Program Outlook. To access assessment documents, meeting materials or other supporting documents related to this assessment, see the list of Chemical Documents. For more information about the development process, visit the IRIS Process page.
Chemical Documents
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Quick Check

Related Links
Tumor Sites
Chemical Structure
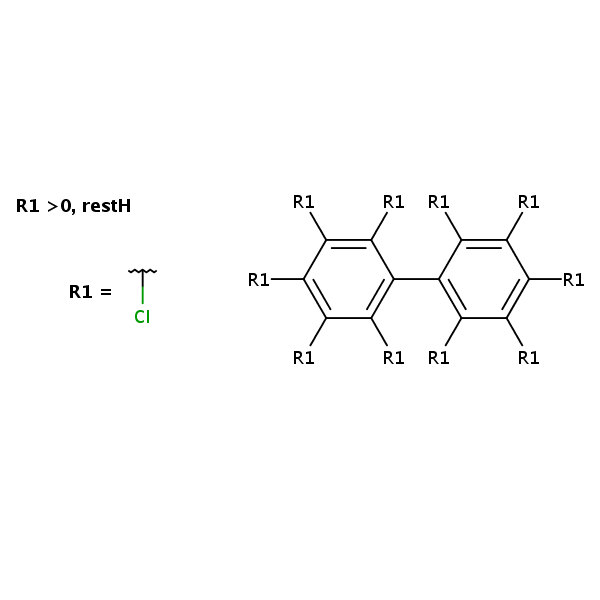
Synonyms
- Aroclor
- Aroclor 1221
- Aroclor 1232
- Aroclor 1242
- Aroclor 1248
- Aroclor 1254
- Aroclor 1260
- Aroclor 1262
- Aroclor 1268
- Aroclor 2565
- Aroclor 4465
- Aroclor 5442
- Biphenyl, polychloro-
- Chlophen
- Chlorextol
- Chlorinated biphenyl
- Chlorinated diphenyl
- Chlorinated diphenylene
- Chloro 1,1-biphenyl
- Chloro biphenyl
- Clophen
- Dykanol
- Fenclor
- Inerteen
- Kanechlor
- Kanechlor 300
- Kanechlor 400
- Montar
- Noflamol
- PCB
- PCBs
- Phenochlor
- Phenoclor
- Polychlorinated biphenyl
- Polychlorinated biphenyls
- Polychlorinated biphenyls (PCBs)
- Polychlorobiphenyl
- Pyralene
- Pyranol
- Santotherm
- Santotherm fr
- Sovol
- Therminol fr-1
- UN 2315
- 1336-36-3

