Naphthalene
CASRN 91-20-3 | DTXSID8020913
- Toxicological Review (PDF) (116 pp, 565 K)
- IRIS Summary (PDF) (32 pp, 102 K)
- Status: Naphthalene is in step 1 at this time; see Quick Check.
On this page:
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (32 pp, 102 K) Last Updated: 09/17/1998
| System | RfD (mg/kg-day) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Other | 2 x 10 -2 | Decreased mean terminal body weight in males |
NOAEL
(ADJ):
7.1
x 101
mg/kg-day |
3000 | Low |
Reference Concentration for Inhalation Exposure (RfC) (PDF) (32 pp, 102 K) Last Updated: 09/17/1998
| System | RfC (mg/m3) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Nervous, Respiratory | 3 x 10 -3 | Nasal effects: hyperplasia and metaplasia in respiratory and olfactory epithelium, respectively |
LOAEL
(HEC):
9.3
mg/m3 |
3000 | Medium |
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(32 pp, 102 K)
Last Updated: 09/17/1998
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| C (Possible human carcinogen) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 1986) |
| Carcinogenic potential cannot be determined | Proposed Guidelines for Carcinogen Risk Assessment (U.S. EPA, 1996) |
- Using criteria of the 1986 Guidelines for Carcinogen Risk Assessment, naphthalene is classified in Group C, a possible human carcinogen. This is based on the inadequate data of carcinogenicity in humans exposed to naphthalene via the oral and inhalation routes, and the limited evidence of carcinogenicity in animals via the inhalation route. Using the 1996 Proposed Guidelines for Carcinogen Risk Assessment, the human carcinogenic potential of naphthalene via the oral or inhalation routes "cannot be determined" at this time based on human and animal data; however, there is suggestive evidence (observations of benign respiratory tumors and one carcinoma in female mice only exposed to naphthalene by inhalation [NTP, 1992a]). Additional support includes increase in respiratory tumors associated with exposure to 1-methylnaphthalene.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (32 pp, 102 K)
Not assessed under the IRIS Program.
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (32 pp, 102 K)
Not assessed under the IRIS Program.
Program Outlook Details
| Public Assessment Materials | Date |
|---|---|
| Problem Formulation Materials/IRIS Assessment Plan | Jul-2018 |
| Preliminary Assessment Materials/Systematic Review Protocol | Mar-2023 |
| Public Comment | TBD |
| External Peer Review | TBD |
| Post Final Assessment | TBD |
Note: Any future dates displayed in the table above should be considered estimates and are subject to change. Once the external peer review is complete, estimated dates for release of the final assessment will be published. For the latest information on the status of this assessment, please refer to the IRIS Program Outlook. To access assessment documents, meeting materials or other supporting documents related to this assessment, see the list of Chemical Documents. For more information about the development process, visit the IRIS Process page.
Chemical Documents
Mar 2023: Protocol for the Naphthalene IRIS Assessment (Preliminary Assessment Materials) (Report)
Apr 2019: IRIS Program Outlook April 2019-Suspended/Discontinued Assessment (Other)
Jul 2018: IRIS Assessment Plan for Naphthalene (2018, Scoping and Problem Formulation Materials) (Report)
Jul 2014: IRIS Toxicological Review of Naphthalene (2014, Scoping and Problem Formulation Materials) (Report)
Aug 2005: Research Needs Related to Naphthalene Assessment (2005, Workshop) (Report)
Jul 2004: IRIS Toxicological Review of Naphthalene (2004, External Review Draft, Update) (Report)
Aug 1998: IRIS Toxicological Review of Naphthalene (1998, Final) (Report)
Mar 1986: Health and Environmental Effects Profile for Naphthalene (1986) (Report)
Sep 1984: Health Effects Assessment for Naphthalene (Report)
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Quick Check

Critical Effects
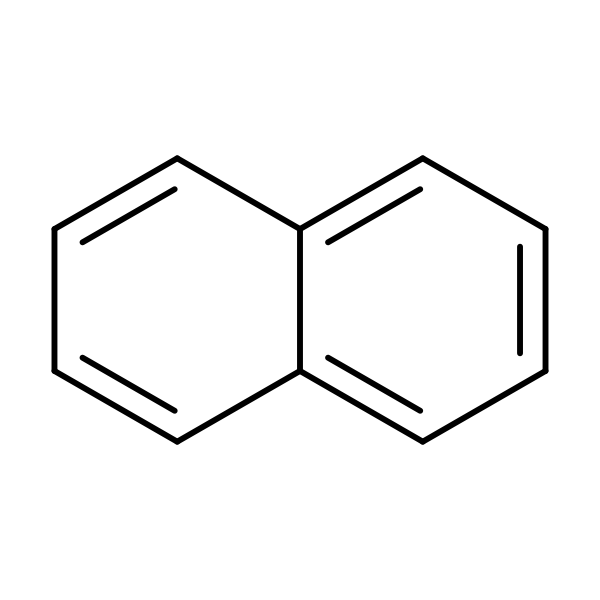
Chemical Structure
Synonyms
- Albocarbon
- Dezodorator
- EPA Pesticide Chemical Code 055801
- HSDB 184
- Moth Balls
- Moth Flakes
- NCI-C52904
- NSC 37565
- Naftalen [Polish]
- Naftaleno [Spanish]
- Naphtalene [French]
- Naphthalene
- Naphthalene
- Naphthalin
- Naphthaline
- Naphthene
- Napthalene, molten
- RCRA Waste Number U165
- Tar Camphor
- UN 1334
- UN 2304
- White Tar
- caswell No. 587
- 91-20-3