Naphthalene
CASRN 91-20-3 | DTXSID8020913
- Toxicological Review (PDF) (116 pp, 551.8 KB, about PDF)
- IRIS Summary (PDF) (32 pp, 99.6 KB, about PDF)
- Status: naphthalene is in step 1 at this time.
IRIS Assessment Plan for Naphthalene (2018, Scoping and Problem Formulation Materials)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
In July 2018, EPA released the IRIS Assessment Plan (IAP) for Naphthalene for public review and comment. An IRIS Assessment Plan (IAP) communicates to the public the plan for assessing each individual chemical and includes summary information on the IRIS Program’s scoping and initial problem formulation; objectives and specific aims for the assessment; the PECO (Populations, Exposures, Comparators, and Outcomes) criteria that outlines the evidence considered most pertinent to the assessment; and identification of key areas of scientific complexity. The PECO provides the framework for developing literature search strategies and inclusion/exclusion criteria, particularly with respect to evidence stream (i.e., human, animal, mechanistic), exposure measures and outcome measures.Background
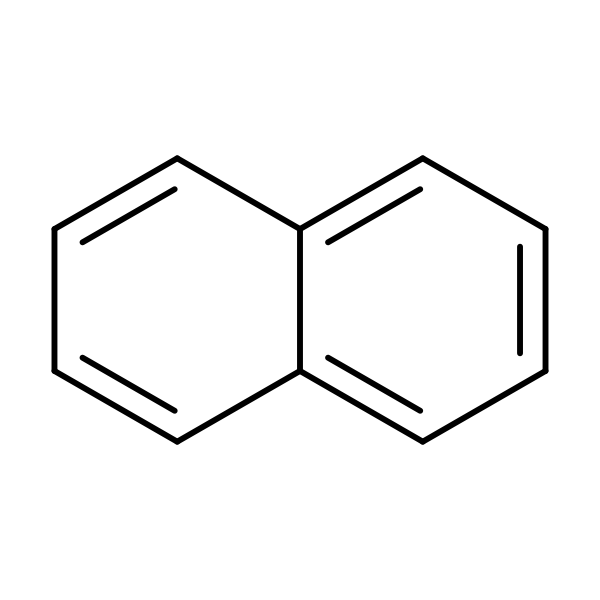
Naphthalene (CAS no. 91-20-3) is a bicyclic aromatic hydrocarbon with the chemical formula C10H8 and a molecular weight of 128.16. Pure naphthalene is a white, water-insoluble solid at room temperature with a vapor pressure of 0.087 mmHg (U.S. EPA, 1987; ATSDR,1993). Naphthalene is produced by distillation and fractionation of either petroleum or coal tar.Naphthalene’s principal use is as an intermediate in the production of phthalic anhydride. Phthalic anhydride is important in the manufacturing of phthalate plasticizers, resins, dyes as well as insect and animal repellents. Naphthalene is also used in the manufacture of synthetic leather tanning agents and the insecticide carbaryl. Naphthalene has been used as a moth repellent and as a deodorizer for diaper pails and toilets (ATSDR, 1993; U.S. EPA, 1980, 1987).
| Date | Description |
|---|---|
| Aug 1998 | EPA revised the IRIS Summary and Toxicological Review of Naphthalene and released an external review draft for public comment. |
| Jul 2004 | EPA released a revised IRIS Summary and Toxicological Review of Naphthalene (External Review Draft) for public comment and hosted an peer review panel meeting. |
| Apr 2005 | EPA hosted a Peer Consultation Workshop on research needs related to the mode of action of inhalation carcinogenicity on April 7, 2005. |
| Aug 2005 | EPA released the document Research Needs Related to Naphthalene Assessment summary report from the workshop. |
| Jul 2014 | EPA released the IRIS Toxicological Review of Naphthalene (Scoping and Problem Formulation Materials) for discussion at a public science meeting held on September 3-4, 2014 to provide the opportunity for the public to give input on the developing draft assessment. |
| Jul 2018 | EPA released the IRIS Assessment Plan of Naphthalene (Scoping and Problem Formulation) for a 30-day public comment period. An August 23, 2018 IRIS Public Science Webinar to discuss the document was also announced. [Federal Register Notice Jul 5, 2018] |
| Aug 2018 | EPA has announced that the August 23, 2018 IRIS Public Science Meeting was postponed until the IRIS Program identified suitable public science meeting dates in the near future. Stakeholders wishing to provide written comments to the IRIS Program were invited to continue to do so through the docket on Regulations.gov until September 5, 2018. |
| Apr 2019 | EPA suspended the assessment in fiscal year 2019. It may be restarted as Agency priorities change. |
| Mar 2021 | The IRIS assessment of naphthalene was released from suspension and announced in the June 2021 IRIS Program Outlook. |
| Sep 2021 | EPA updated the IAP and posted an errata sheet outlining the changes that were made to the draft assessment and posted on this site on Sep 30, 2021. |
| Sep 2021 | EPA announced the rescheduling of the IRIS Public Science Meeting for Naphthalene to be held on November 9, 2021 to discuss the key science issues identified in the 2018 IRIS Assessment Plan (IAP). |
Download(s)
This download(s) is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy.
- IRIS Assessment Plan for Naphthalene (Scoping and Problem Formulation Materials) (PDF) (23 pp, 441.4 KB, about PDF)
- Errata Sheet (PDF) (1 pp, 134.8 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
- Docket No: EPA-HQ-ORD-2014-0527
- Summary Review of Health Effects Associated with Naphthalene: Health Issue Assessment (1987)
- IRIS Toxicological Review of Naphthalene (1998, Final)
- IRIS Toxicological Review of Naphthalene (2004, External Review Draft, Update)
- Final Report from the External Peer Review of the IRIS Reassessment of the Inhalation Carcinogenicity of Naphthalene (2004)
- Research Needs Related to Naphthalene Assessment (2005, Workshop)
- IRIS Toxicological Review of Naphthalene (2014, Scoping and Problem Formulation Materials)
- IRIS Program Outlook April 2019-Suspended/Discontinued Assessment
Federal Register Notices
Quick Check

Critical Effect Systems
Chemical Structure for
Naphthalene
Synonyms
- Albocarbon
- Dezodorator
- EPA Pesticide Chemical Code 055801
- HSDB 184
- Moth Balls
- Moth Flakes
- NCI-C52904
- NSC 37565
- Naftalen [Polish]
- Naftaleno [Spanish]
- Naphtalene [French]
- Naphthalene
- Naphthalene
- Naphthalin
- Naphthaline
- Naphthene
- Napthalene, molten
- RCRA Waste Number U165
- Tar Camphor
- UN 1334
- UN 2304
- White Tar
- caswell No. 587
- 91-20-3