Naphthalene
CASRN 91-20-3 | DTXSID8020913
- Toxicological Review (PDF) (116 pp, 551.8 KB, about PDF)
- IRIS Summary (PDF) (32 pp, 99.6 KB, about PDF)
- Status: naphthalene is in step 1 at this time.
Research Needs Related to Naphthalene Assessment (2005, Workshop)
On this page:
Alert
Notice - This site contains archived material(s)
Archive disclaimer
Archived files are provided for reference purposes only.
The file was current when produced, but is no longer maintained and may now be outdated.
Persons with disabilities having difficulty accessing archived files may contact the IRIS Webmaster for assistance.
Please use the contact us form if you need additional support.
Overview
EPA has announced the release of the final report from the 2005 peer consultation workshop which sought expert opinion on research needs related to the mode of action of the inhalation carcinogenicity of naphthalene. This report is a summary of the main points of presentations and highlights discussions among the participants.Workshop Details
The U.S. Environmental Protection Agency (EPA) announced via Federal Register Notice March 8, 2005 [FRL-7882-1], a workshop to seek expert opinion on the research needs related to the mode of action of the inhalation carcinogenicity of naphthalene. EPA's IRIS program is developing a health assessment on naphthalene carcinogenicity. A draft assessment was peer reviewed in July 2004. One of the topics discussed at the peer review workshop was the need for further research to characterize naphthalene's carcinogenic mode of action. The Agency has decided to hold a peer consultation workshop on this topic before determining a course of action on the IRIS assessment of naphthalene. The workshop will be held on April 7, 2005 at the Graduate School of Public Health, 130 DeSoto Street, Room 109 Parran Hall, University of Pittsburgh, Pittsburgh, PA 15261.
Background
Naphthalene is used as a moth repellant and toilet bowl deodorant, in the manufacture of numerous chemicals, in veterinary antiseptics to irrigate wounds, and as an external medication to control lice on livestock. It is a constituent of creosote, tar and fossil fuels and is released during the combustion of organic material and from automobile exhaust.| Date | Description |
|---|---|
| 1998 | EPA revised the IRIS Summary and Toxicological Review of Naphthalene and released an external review draft for public comment. |
| Jun 2004 | EPA revised the IRIS Summary and Toxicological Review of Naphthalene and released an external review draft for public comment. |
| Jul 2004 | EPA hosted an External Peer Review Panel Meeting of a revised inhalation carcinogenicity assessment. |
| Mar 2005 | EPA hosted a Peer Consultation Workshop on research needs related to the mode of action of inhalation carcinogenicity which was held on April 7, 2005. |
| Aug 2005 | EPA released the Final Peer Consultation Report from the workshop. |
Status
Following the External Peer Review, the final assessment is scheduled to be posted to the IRIS database.Download(s)
This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
- Peer Consultation Workshop Charge Statement (PDF) (1 pp, 19.5 KB, about PDF)
- CONSOLIDATED COMMENTS FROM THE EXTERNAL PEER REVIEW WORKSHOP (FINAL) (PDF) (39 pp, 139.6 KB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
Federal Register Notices
Quick Check

Critical Effect Systems
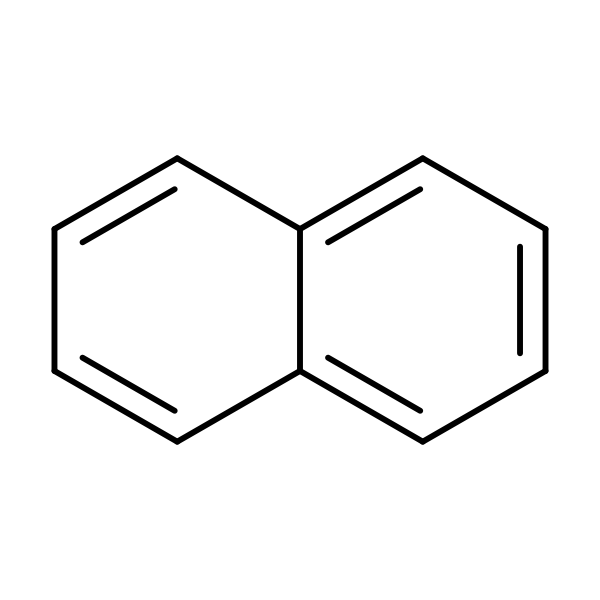
Chemical Structure for
Naphthalene
Synonyms
- Albocarbon
- Dezodorator
- EPA Pesticide Chemical Code 055801
- HSDB 184
- Moth Balls
- Moth Flakes
- NCI-C52904
- NSC 37565
- Naftalen [Polish]
- Naftaleno [Spanish]
- Naphtalene [French]
- Naphthalene
- Naphthalene
- Naphthalin
- Naphthaline
- Naphthene
- Napthalene, molten
- RCRA Waste Number U165
- Tar Camphor
- UN 1334
- UN 2304
- White Tar
- caswell No. 587
- 91-20-3