1,2,4-Trimethylbenzene
CASRN 95-63-6 | DTXSID6021402
- Toxicological Review (PDF) (150 pp, 2.5 MB, about PDF)
- IRIS Executive Summary (PDF) (10 pp, 315.4 KB, about PDF)
- Supplemental Information on the IRIS Toxicological Review of Trimethylbenzene
IRIS Toxicological Review of Trimethylbenzenes (Final Report)
On this page:
Overview
EPA has finalized the Integrated Risk Information System (IRIS) Assessment of Trimethylbenzenes (TMBs). This assessment addresses the potential non-cancer and cancer human health effects from long-term exposure to TMBs. Now final, this assessment will be the first IRIS assessment for TMBs that may be used by EPA’s program and regional offices to inform decisions to protect human health.Background
Trimethylbenzenes (TMBs) are a commercially available mixture of three individual isomers: 1,2,3-TMB, 1,2,4-TMB, and 1,3,5-TMB. TMB isomers are produced during petroleum refining and production of aromatic hydrocarbons with nine carbons (i.e., C9 aromatic fraction). As the vast majority of the C9 fraction is used as a component of gasoline, vehicle emissions are expected to be the major anthropogenic source of TMBs. TMBs are volatile hydrocarbons, and humans are thus exposed to these isomers primarily through breathing air containing TMB vapors, although ingestion through food or drinking water is also possible.Effects on the nervous, respiratory, and hematological (i.e., blood) systems have been reported in occupationally- and residentially-exposed humans, but these effects were observed following exposure to complex mixtures containing TMB isomers, thus making it difficult to determine the contribution of each TMB isomer to the observed health effects. Health effects that are roughly analogous to those seen in humans have been observed in animals exposed to the individual isomers. Effects on the nervous system, including cognitive effects and decreased pain sensitivity, are the most widely observed effects in animals. Effects on other systems, including the respiratory and hematological systems, have also been observed in animals. Both 1,2,4-TMB and 1,3,5-TMB have been observed to elicit effects on pregnant animals and developing fetuses, but at exposure levels greater than those that cause effects on the nervous system. There is inadequate information to evaluate the carcinogenicity of TMBs.
| Date | Description |
|---|---|
| Jan 2012 | EPA submits the draft assessment for Interagency Science Consultation. In February, EPA hosted an interagency science consultation meeting on the review of the draft Toxicological Review of Trimethylbenzenes. |
| Jun 2012 | EPA released the external peer review draft of the trimethylbenzenes assessment for public review and comment. Additionally, the interagency science consultation draft, comments from reviewers, and EPA's responses to selected major interagency comments were also released (see "related links"). [Federal Register Notice June 29, 2012] |
| Aug 2012 | EPA conducts public listening session on the draft assessment. |
| Aug 2013 | EPA releases the revised draft assessment for external peer review by EPA's Science Advisory Board (SAB) Chemical Assessment Advisory Committee (CAAC). |
| Jun 2014 | EPA's SAB CAAC convenes to discuss the external review draft of the IRIS assessment. |
| Jun 2016 | EPA submits the revised interagency science discussion draft of the Toxicological Review of Trimethylbenzenes for final Agency review and Interagency science discussion. |
| Sep 2016 | EPA posted the final IRIS Toxicological Review of Trimethylbenzenes to the IRIS database. |
Download(s)
This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
- IRIS Executive Summary of Trimethylbenzes (PDF) (10 pp, 318.4 KB, about PDF)
- IRIS Toxicological Review of Trimethylbenzes (Final Report) (PDF) (150 pp, 2.7 MB, about PDF)
- IRIS Toxicological Review of Trimethylbenzes (Final Report) -- Supplemental Information (PDF) (347 pp, 8.8 MB, about PDF)
If you have a disability and the format of any material on our web pages interferes with your ability to access the information, please reach out to us using the Contact Us about IRIS form for assistance. To enable us to respond in a manner most helpful to you, please indicate the nature of the accessibility problem, the web address of the requested material, your preferred format in which you want to receive the material (electronic format (ASCII, etc.), standard print, large print, etc.), and your contact information.
Document Related Link(s)
- IRIS Toxicological Review of Trimethylbenzenes (External Review Draft)
- IRIS Toxicological Review of Trimethylbenzenes (Interagency Science Consultation Draft)
- IRIS Toxicological Review of Trimethylbenzenes (Revised External Review Draft)
- IRIS Toxicological Review of Trimethylbenzenes (Interagency Science Discussion Draft)
Related Links
Critical Effect Systems
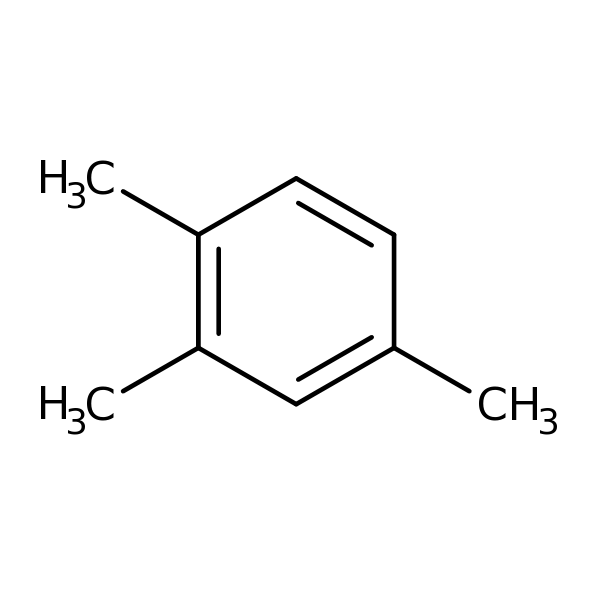
Chemical Structure for
1,2,4-Trimethylbenzene
Synonyms
- asymmetrical trimethylbenzene
- mesitylene
- methylxylene
- pseudocumene
- psi-Cumene