Chromium(VI)
CASRN 18540-29-9 | DTXSID7023982
- Toxicological Review (PDF) (538 pp, 10.09 M)
- IRIS Summary (PDF) (14 pp, 473 K)
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (14 pp, 473 K) Last Updated: 08/01/2024
| System | RfD (mg/kg-day) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Gastrointestinal | 9 x 10 -4 | Hyperplasia in small intesting of female mice (chronic exposure study). |
HED
:
9.11
x 10-2
mg/mg-day |
100
MF: None |
Medium-High |
Reference Concentration for Inhalation Exposure (RfC) (PDF) (14 pp, 473 K) Last Updated: 08/01/2024
| System | RfC (mg/m3) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Respiratory | 3 x 10 -5 | Ulcerated nasal septum in humans |
LOAEL
:
3.4
x 10-3
mg/m3 |
100
MF: None |
Medium |
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(14 pp, 473 K)
Last Updated: 08/01/2024
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| Carcinogenic to humans (Inhalation route) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005) |
| Likely to be carcinogenic to humans (Oral route) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005) |
- Under EPA’s Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005), Cr(VI) is “likely to be carcinogenic” via oral exposure based on sufficiently supported [laboratory] animal studies relevant to humans. This assessment maintains the previous conclusion (U.S. EPA, 1998) that Cr(VI) is “carcinogenic to humans” via inhalation exposure.
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (14 pp, 473 K)
Oral Slope Factor:
0.27
per mg/kg-day
Extrapolation Method: HEDs calculated using BW3/4 scaling
Tumor site(s): Gastrointestinal
Tumor type(s): Squamous cell carcinoma or squamous cell papilloma. (NTP, 2008)
Note: EPA has concluded that Cr(VI) is carcinogenic by a mutagenic mode of action. Thus, based on the EPA cancer guidelines (2005), the oral slope factor (OSF) addressing lifetime exposure includes application of age-dependent adjustment factors (ADAFs). The OSF is recommended for lifetime exposures. An adult-based cancer slope factor of 0.16 per mg/kg-day is also provided. This adult-based cancer slope factor can be used instead of the OSF when assessing cancer risk associated with exposure scenarios that don’t include early life (< 16 years of age) or when other calculations by the user are necessary (e.g., when applying ADAFs to age-specific exposure estimates).
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (14 pp, 473 K)
Inhalation Unit Risk:
0.018
per µg/m3
Extrapolation Method: Linear extrapolation from LEC01 derived from Cox proportional hazard model with 5 year lag using lifetable analysis.
Tumor site(s): Respiratory
Tumor type(s): Lung cancer
Note: EPA has concluded that Cr(VI) is carcinogenic by a mutagenic mode of action. Thus, based on the EPA cancer guidelines (2005), the inhalation unit risk (IUR) addressing lifetime exposure includes application of age-dependent adjustment factors (ADAFs). The IUR is recommended for lifetime exposures. An adult-based cancer inhalation unit risk of 0.011 per µg/m3 is also provided. This adult-based cancer inhalation unit risk can be used instead of the IUR when assessing cancer risk associated with exposure scenarios that don’t include early life (< 16 years of age) or when other calculations by the user are necessary (e.g., when applying ADAFs to age-specific exposure estimates).
Organ/System-Specific RfDs
| System | RfD (mg/m3) | Basis | PoD | UF | Confidence | Notes |
|---|---|---|---|---|---|---|
| Gastrointestinal | 9 x 10-4 | Hyperplasia in small intesting of female mice (chronic exposure study). |
HED
:
9.11 x 10-2 mg/mg-day |
100 | Medium-High | LOAEL |
| Hepatic | 7 x 10-4 | Chronic inflammation in female rats. |
HED
:
0.0669 mg/mg-day |
100 | Medium-High | LOAEL |
| Developmental | 0.07 | Decreased postnatal growth in F1 offspring in mice (continuous breeding study) |
HED
:
0.700 mg/mg-day |
10 | Low | NOAEL |
| Hematologic | 0.01 | Decreased hemoglobin in male rats (short-term exposure data). |
HED
:
0.126 mg/mg-day |
10 | Medium | BMDL 1 SD |
Chemical Documents
Aug 2024: IRIS Toxicological Review of Hexavalent Chromium (Final Report, 2024) (Report)
Oct 2022: IRIS Toxicological Review of Hexavalent Chromium (External Review Draft, 2022) (Report)
Sep 2013: Hexavalent Chromium Workshops (2013) (Other)
Sep 2010: IRIS Toxicological Review of Hexavalent Chromium (2010 External Review Draft) (Report)
Sep 1998: IRIS Toxicological Review of Chromium VI (1998 Final) (Report)
Apr 1990: Noncarcinogenic Effects of Chromium: Update to Health Assessment Document (Report)
Sep 1984: Health Effects Assessment for Hexavalent Chromium (1984 Final) (Report)
Aug 1983: Health Assessment Document for Chromium (1983 Final) (Report)
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Critical Effects
Tumor Sites
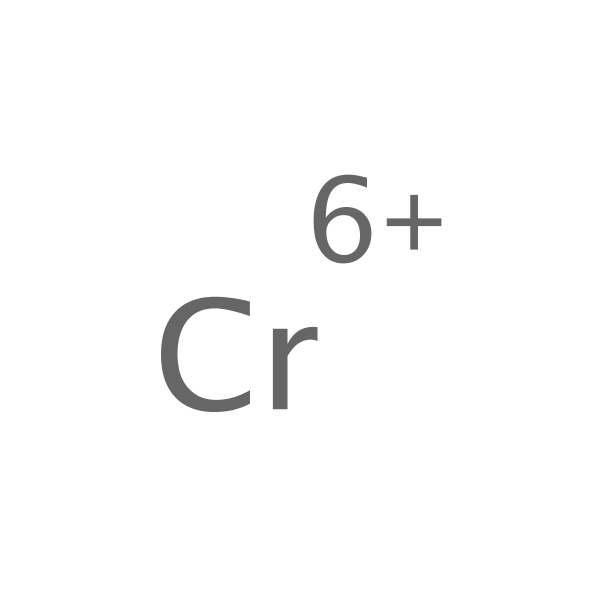
Chemical Structure
Synonyms
- Chromic ion
- Chromium
- Chromium (VI)
- Chromium (VI) ion
- Chromium, ion
- 18540-29-9
- 7440-47-3