Formaldehyde
CASRN 50-00-0 | DTXSID7020637
- Toxicological Review (PDF) (927 pp, 17.11 M)
- IRIS Executive Summary (PDF) (10 pp, 461 K)
On this page:
Noncancer Assessment
Reference Dose for Oral Exposure (RfD) (PDF) (10 pp, 461 K) Last Updated: 09/01/1990
| System | RfD (mg/kg-day) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Urinary, Gastrointestinal, Other | 2 x 10 -1 | Reduced weight gain, histopathology in rats |
NOAEL
:
1.5
x 101
mg/kg-day |
100 | Medium |
Reference Concentration for Inhalation Exposure (RfC) (PDF) (10 pp, 461 K) Last Updated: 08/19/2024
| System | RfC (mg/m3) | Basis | PoD | Composite UF | Confidence |
|---|---|---|---|---|---|
| Respiratory | 7 x 10 -3 | Decreased pulmonary function, prevalence of current asthma or degree of asthma control, and allergic conditions in children (See Note) | High |
Note: The RfC is the median of three organ specific RfCs (osRfCs), which may individually be used in cumulative risk scenarios — (1) 0.008 mg/m3 using a composite UF (UFC) = 3, the osRfC for allergic conditions based on rhinoconjunctivitis prevalence (Annesi-Maesano et al., 2012); (2) 0.007 mg/m3 using a UFC = 3, the osRfC for pulmonary function based on decreased peak expiratory flow rate (Krzyzanowkski et al., 1990); and (3) 0.006 mg/m3, the osRfC for prevalence of current asthma or degree of asthma symptom control, which is itself the median of three values: 0.01 mg/m3 using a UFC = 3 (Annesi-Maesano et al., 2012); 0.006 mg/m3 using a UFC = 10 (Krzyzanowkski et al., 1990); and 0.004 mg/m3 using a UFC = 3 (Venn et al., 2003). See Section 5.1 of the IRIS Toxicological Review for more information.
Cancer Assessment
Weight of Evidence for Cancer (PDF)
(10 pp, 461 K)
Last Updated: 08/19/2024
| WOE Characterization | Framework for WOE Characterization |
|---|---|
| Carcinogenic to humans (Inhalation route) | Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005) |
- Under EPA’s Guidelines for Carcinogen Risk Assessment (U.S. EPA, 2005), formaldehyde is “carcinogenic to humans by the inhalation route of exposure”. This conclusion is independently supported by three sets of evidence, namely evidence integration (aka “weight of evidence”) judgments that the evidence demonstrates that formaldehyde inhalation causes nasopharyngeal cancer, sinonasal cancer, and myeloid leukemia, in exposed humans (see the IRIS Toxicological Review for details on the human, animal, and/or mechanistic evidence that supports each of these three judgments).
- This may be a synopsis of the full weight-of-evidence narrative.
Quantitative Estimate of Carcinogenic Risk from Oral Exposure (PDF) (10 pp, 461 K)
Not Assessed under the IRIS Program.
Quantitative Estimate of Carcinogenic Risk from Inhalation Exposure (PDF) (10 pp, 461 K)
Inhalation Unit Risk:
1.1
x 10-5
per µg/m3
Extrapolation Method: Based on nasopharyngeal cancer (NPC) incidence (Beane Freeman, 2013), linear low-dose extrapolation from the 95% lower bound on the exposure level associated with the extra risk level serving as the benchmark response and application of age-dependent adjustment factors (ADAFs) due to an operant mutagenic mode-of-action (MOA) for nasal cancers (see Section 3.2.5 of the Toxicological Review). Confidence in the IUR is Medium (see Section 5.2 of the Toxicological Review).
Tumor site(s): Hematologic, Respiratory
Tumor type(s): Nasopharyngeal cancer, sinonasal cancer, myeloid leukemia
Note: The IUR is based on NPC alone, although it was concluded that the evidence demonstrates that formaldehyde inhalation also causes myeloid leukemia and sinonasal cancer (see Section 5.2 of the Toxicological Review for details). Thus, the IUR may underestimate the actual total cancer risk. Less-than-lifetime exposure scenarios with a very large fraction of exposure during adulthood may not warrant ADAF adjustment, and one may choose to use the unadjusted unit risk estimate of 7.4 × 10-6 per µg/m3 or the adult-based estimate of 6.4 × 10-6 per µg/m3 (see Section 5.2.4 of the Toxicological Review).
Chemical Documents
Aug 2024: IRIS Toxicological Review of Formaldehyde (Inhalation) (Final Report, 2024) (Report)
Jun 2024: IRIS Toxicological Review of Formaldehyde (Interagency Science Discussion Draft, 2024) (Report)
Apr 2022: IRIS Toxicological Review of Formaldehyde-Inhalation (External Review Draft, 2022) (Report)
Jun 2021: IRIS Program Outlook Jun 2021-Unsuspended Assessment (Other)
Apr 2019: IRIS Program Outlook April 2019-Suspended/Discontinued Assessment (Other)
Jun 2010: IRIS Toxicological Review of Formaldehyde (Inhalation) (External Review Draft, 2010) (Report)
May 2010: IRIS Toxicological Review of Formaldehyde (Interagency Science Consultation Draft, 2010) (Report)
Dec 2009: Statistical Inferences from Formaldehyde Dna-Protein Cross-Link Data (Journal)
Sep 1991: IRIS Summary of Formaldehyde (Noncancer, 1991) (Report)
Sep 1990: IRIS Summary of Formaldehyde (Cancer, 1990) (Report)
Sep 1985: Health and Environmental Effects Profile for Formaldehyde (Report)
Other EPA Information
- Human Health Benchmarks for Pesticides (HHBP). This database provides human health benchmarks for pesticides that may be present in drinking water.
- Office of Pesticide Programs Pesticide Chemical Search. This database provides links to health effects information and registration status for pesticides.
- Chemistry Dashboard. This database provides information on chemical structures, experimental and predicted physicochemical, and toxicity data.
Related Links
Critical Effects
Tumor Sites
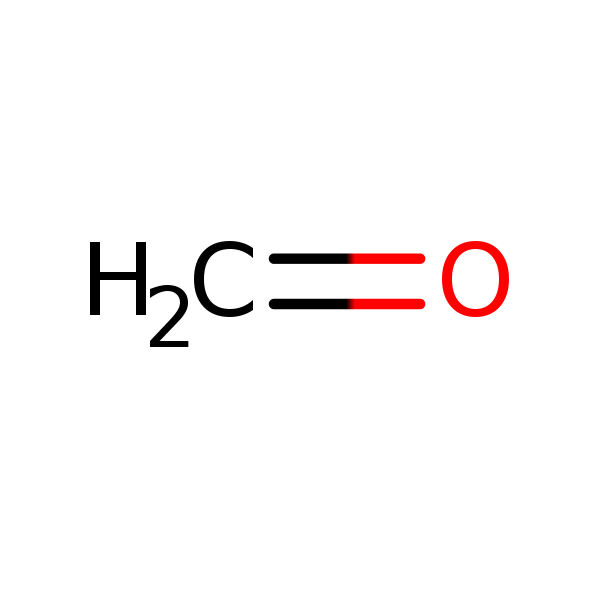
Chemical Structure
Synonyms
- Aldehyd mravenci (Czech)
- Aldehyde formique (French)
- Aldeide formica (Italian)
- BFV
- FA
- Formaldehyd (Czech, Polish)
- Formaldehyde
- Formaldehyde solution (DOT)
- Formalin
- Formalith
- Formic aldehyde
- Formol
- Fyde
- Hoch
- Ivalon
- Karsan
- Lysoform
- Methanal
- Methyl aldehyde
- Methylene glycol
- Methylene oxide
- Morbicid
- NCI-C02799
- Oplossingen (Dutch)
- Oxomethane
- Oxymethylene
- Paraform
- Polyoxymethylene glycols
- RCRA Waste Number U122
- Superlysoform
- UN 1198 (DOT)
- UN 2209 (DOT)
- 50-00-0